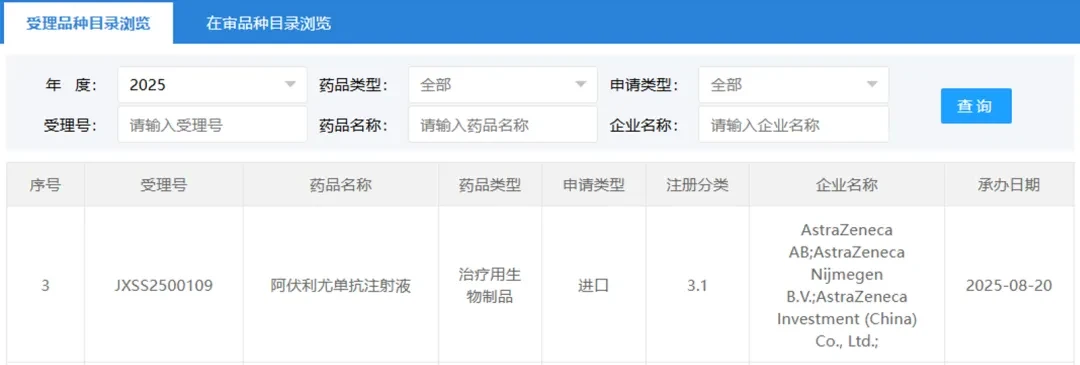
AstraZeneca files for approval in China for new systemic lupus erythematosus drug
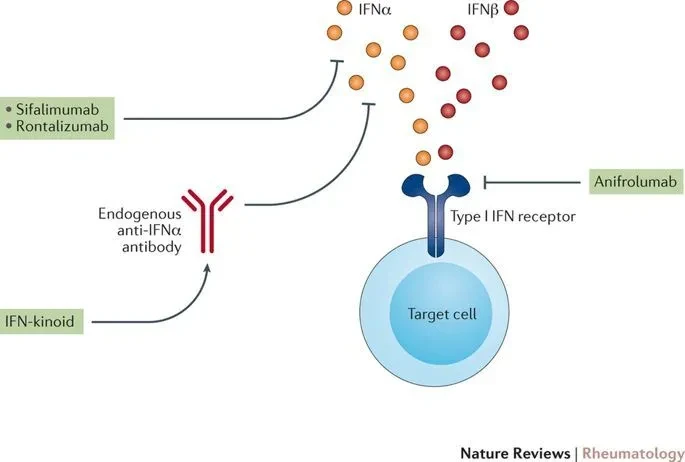
August 2021,AvelumabFirst approved by the FDA for the treatment of adult patients with moderate to severe systemic lupus erythematosus who are receiving standard therapy. Data from multiple registrational clinical studies (includingPhase III TULIP-1, TULIP-2 studies and Phase II MUSE study) indicates that, compared with placebo, on the basis of standard therapy,AvelumabGroup more patients achieving organ system (Including skin and joints) overall disease activity reduction, along with oral corticosteroids (OCS) continues to decline in use.
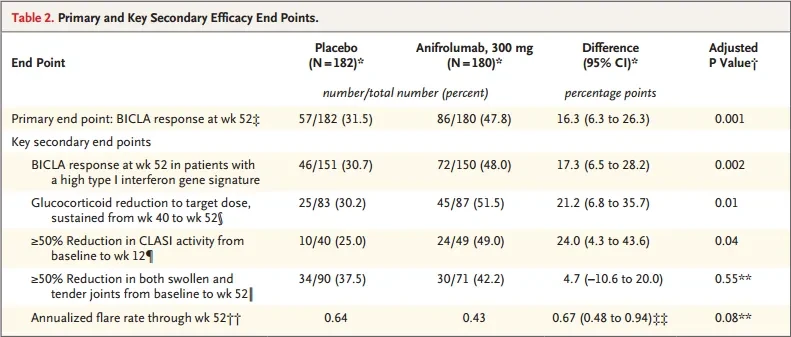
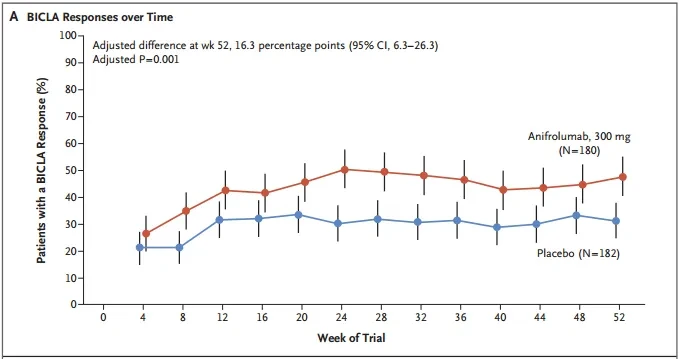
In the TULIP-2 trial, avacopan significantly improved the BILAG-based Composite Lupus Assessment (BICLA).
According to the医药魔方database, two other domestic IFNAR-1 monoclonal antibodies have entered the clinical stage, developed by GenScript Biotech and Quanxian Biopharma, respectively.
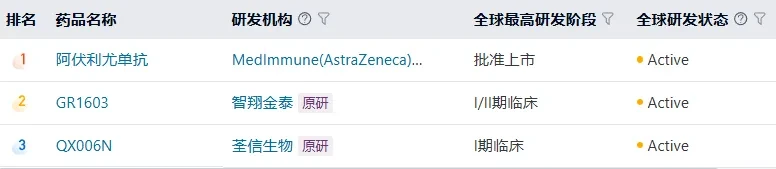
If Avelumab is approved smoothly, it is expected to benefit moreprovides a new treatment option for patients with systemic lupus erythematosus.
