Zenitar completes ~¥600M Series C financing to advance highly differentiated small-molecule therapies
Recently, Zenitar announced the successful completion of an approximately 600 million Series C financing round. This round was jointly led by True Light Capital, a wholly-owned subsidiary of Temasek, and Qiming Venture Partners, with participation from Tencent Investment, Lanchi Ventures, C&D Emerging Industry Equity Investment, and Shanghai Sci-Tech Innovation Center Capital. Existing shareholders, including 5Y Capital, Huajin Capital, Guosheng Capital, and Sichuan Science and Technology Innovation Investment, continued to provide support.
The funds raised in this round will primarily be allocated to global clinical research and development, pipeline expansion, and technology platform upgrades. Specifically, this includes:
Global Clinical Development: Accelerate the global clinical development of two hematology/oncology drug candidates currently in Phase III registrational studies, expand their indications, and initiate overseas clinical trials for multiple candidates in immunology, inflammation, and central nervous system diseases to establish an international market presence.
Pipeline Expansion: Continuously enrich and upgrade the innovative drug pipeline through both indication expansion of existing candidates and the development of novel molecules targeting new pathways with differentiated designs.
Technology Platform Enhancement: Strengthen the synergistic capabilities of three core technology platforms—ZeniFold for deep structural biology insights, ZeniMind for AI-driven drug discovery and design, and ZeniScreen for clinically relevant disease screening models—to systematically enhance end-to-end drug R&D capabilities.
Partial Display of Zenitar's Clinical and Preclinical Product Portfolio
Founded in 2019, Zenitar is a clinical-stage biotechnology company. By integrating structural biology, artificial intelligence, and clinically relevant disease models, Zenitar has established an integrated core technology platform for new drug discovery and development. This has enabled the efficient construction of a diverse and globally competitive R&D pipeline with clear differentiation, dedicated to developing highly differentiated small molecule therapies with "First-in-class" or "Best-in-class" potential.
Zenitar focuses on hematological diseases, oncology, central nervous system (CNS) disorders, and inflammation/immunology (I&I), aiming to create innovative treatment standards or significantly enhance existing therapeutic efficacy for diseases with substantial unmet clinical needs.
Zenitar integrates three platforms—ZeniFold (structural biology platform), ZeniMind (AI-driven drug discovery and design platform), and ZeniScreen (clinically relevant disease model screening platform)—to build a synergistic, "three-in-one" R&D engine. This forms a closed-loop iterative system spanning from target identification and molecular design to efficacy validation. ZeniFold relies on high-precision protein structure analysis to accurately identify drug-binding sites and guide molecular scaffold design. ZeniMind utilizes proprietary AI algorithms to enable intelligent molecular generation and multi-objective parameter optimization. ZeniScreen leverages clinically relevant disease models to systematically conduct efficacy validation and assess clinical translation potential.
Through this closed-loop workflow of "structure elucidation – AI design – disease model validation – structural feedback validation," Zenitar achieves continuous cross-validation and iterative optimization between computational and experimental data. This significantly enhances lead compound discovery efficiency and candidate drug quality, successfully supporting the formation and advancement of multiple small molecule projects in its pipeline.
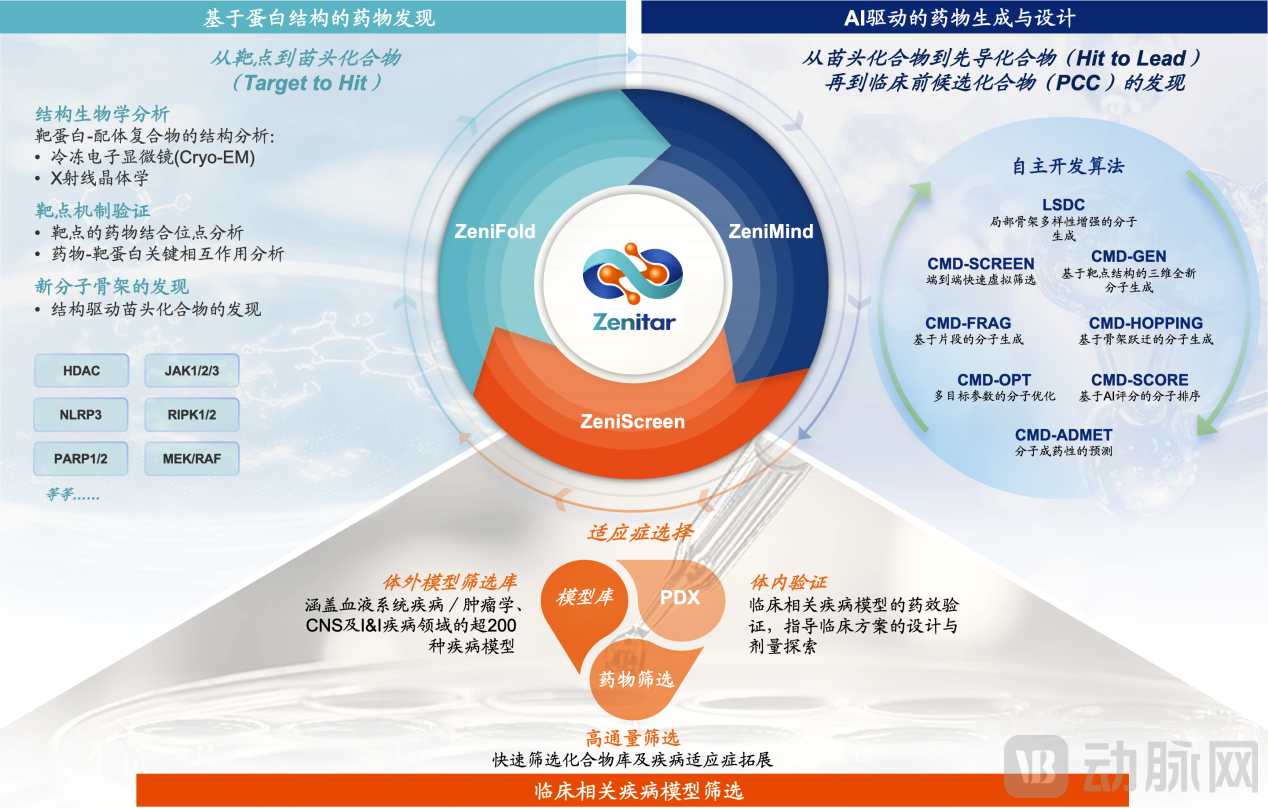
Schematic Diagram of the Collaborative Mechanism of Zenitar's New Drug R&D Platform
