MediLink secures $570M upfront from Roche for B7H3-targeting ADC in major repeat deal
On January 9, MediLink Therapeutics announced that it has entered into a new exclusive licensing agreement with Roche for the YL201 program. The two companies will jointly advance the development and commercialization of this investigational B7H3-targeting antibody-drug conjugate (ADC) for numerous solid tumor indications.
Under the terms of the agreement, MediLink will grant Roche an exclusive license to develop, manufacture, and commercialize YL201 worldwide, excluding the mainland of China, the Hong Kong Special Administrative Region, and the Macau Special Administrative Region. MediLink will receive upfront and near-term milestone payments totaling US$ 570 million, and is eligible to receive additional development, regulatory, and commercial milestone payments, as well as tiered royalties on net sales of YL201 outside of China following its regulatory approval.
YL201: A Validated TMALIN® ADC Advancing in Global Phase III Trials
YL201 is a B7H3-targeting antibody-drug conjugate (ADC) developed based on MediLink's proprietary Tumor Microenvironment-Activatable LINker-payload (TMALIN®) platform, which utilizes a camptothecin-based toxin-linker designed for cleavage within the tumor microenvironment (TME).
Reportedly, traditional ADC drugs still have several limitations. First, for an ADC to exert its therapeutic effect, the antibody component must be internalized by cells, which restricts the selection of suitable antibodies. Furthermore, the release of the ADC's payload involves multiple steps, such as tumor cell endocytosis and lysosomal degradation, each of which may lead to treatment failure or drug resistance. Additionally, ADCs with large molecular sizes often exhibit slow tumor enrichment, poor tissue permeability, and reduced efficacy against tumors with low target antigen expression.
MediLink's TMALIN® technology platform addresses these shortcomings of existing ADC technologies. First, its unique enzymatic cleavage capability enables extracellular payload release within the tumor microenvironment. Regardless of the antibody's endocytic potential, the resulting ADC maintains high anti-tumor activity, significantly broadening the range of antibody options. Second, the specialized toxin-linker design enables ADC enrichment within the tumor microenvironment, increasing the tumor-to-blood concentration ratio of the payload and providing a higher therapeutic index. Third, the combined enzymatic cleavage and tumor enrichment characteristics lead to substantial payload accumulation within tumor tissue, generating a potent bystander effect that delivers effective anti-tumor activity even in tumors with low or no antigen expression.
Moreover, ADCs constructed using the TMALIN® technology offer several additional advantages: They exhibit extremely high systemic circulation stability, reducing "off-target" toxicity caused by payload release in non-target tissues. They possess excellent solubility and chemical stability, eliminating the reversible Michael addition reaction associated with the maleimide conjugation chemistry used in traditional ADCs. This allows for the production of highly homogeneous ADCs with site-specific, quantitative conjugation (DAR=8). The conjugation process achieves high efficiency (≥90%).
The clinical advancement of YL201 serves as a powerful validation of the TMALIN® platform's potential. Currently, YL201 is under global clinical investigation for various advanced solid tumors. In China, it has entered two Phase III registrational trials for small cell lung cancer (SCLC) and nasopharyngeal carcinoma (NPC). Early clinical data in second-line SCLC patients have demonstrated promising objective response rates and survival benefits. In June 2025, the U.S. FDA granted YL201 Breakthrough Therapy Designation for the treatment of SCLC, following three previously awarded Orphan Drug Designations for SCLC, NPC, and Esophageal Squamous Cell Carcinoma (ESCC).
70% of Pipeline Programs Have Secured Licensing Deals
In fact, this is not the first collaboration between MediLink and Roche. As early as January 2024, the two companies announced a partnership to develop YL211, a next-generation antibody-drug conjugate (ADC) candidate targeting the mesenchymal-epithelial transition factor (c-MET), for the treatment of solid tumors, with a total deal value exceeding US$ 1 billion. According to the terms of the agreement, Roche obtained exclusive global rights to develop, manufacture, and commercialize MediLink's YL211 program. MediLink collaborated with the Roche China Innovation Center (CICoR) to advance YL211 into Phase I clinical trials, with Roche responsible for all subsequent global development and commercialization efforts.
Unlike many one-off or opportunistic licensing deals, this "repeat collaboration" model surpasses a single transaction, demonstrating Roche's strong confidence in the ADC field as well as its high regard for MediLink's overall R&D capabilities, the productivity of its technology platform, and the execution strength of its team. Beyond Roche's "repeat deal," MediLink's official website indicates that 7 out of its 10 pipeline programs have secured licensing agreements.
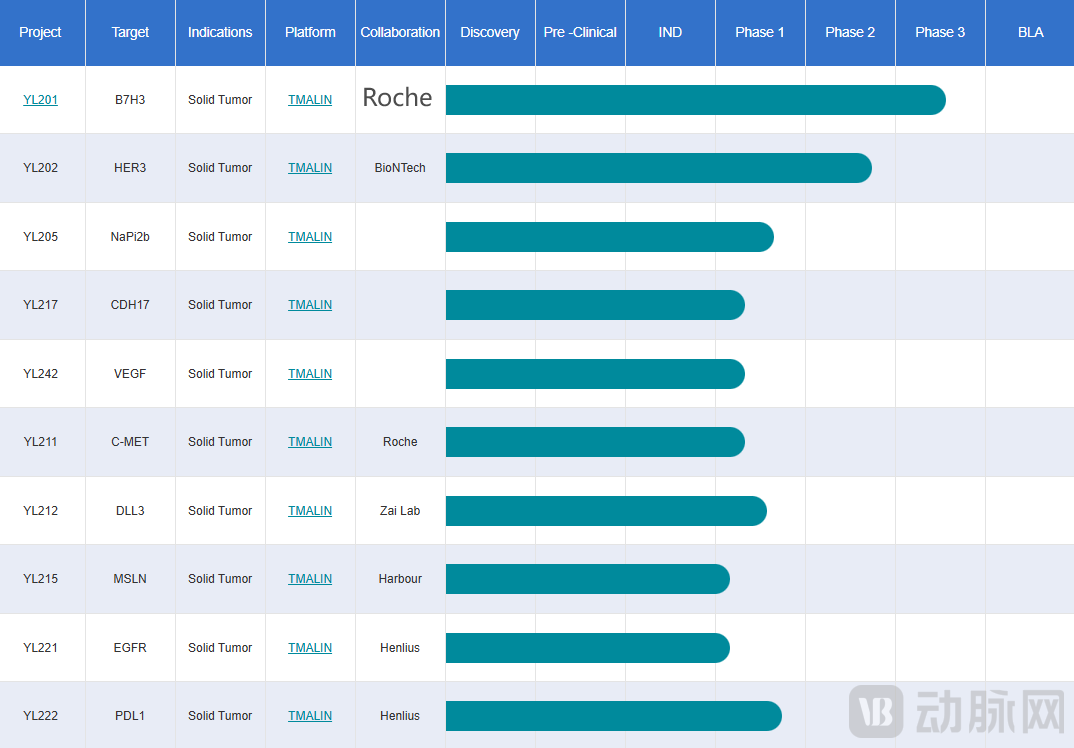 MediLink Therapeutics' R&D Pipeline
MediLink Therapeutics' R&D Pipeline
MediLink's continued recognition within the industry stems from several factors.
From MediLink's own perspective, the advantages of its proprietary TMALIN® technology platform in ADC development have been detailed previously. Furthermore, leveraging this technology platform, MediLink has adopted a differentiated ADC strategy. In its pipeline development, it focuses more on emerging targets such as B7H3, HER3, and DLL3, rather than competing in crowded fields like HER2 to extract incremental value from existing ADC frameworks.
Globally, the ADC field still presents vast blue-ocean opportunities. First, the therapeutic window of current ADC drugs remains relatively narrow, falling short of the safety profile expected of a "magic bullet," indicating significant room for improvement. On the other hand, every aspect of ADC design offers potential for enhancement, including antibody binding specificity, diversity in toxin mechanisms of action, and the specificity of linker cleavage and payload release. Additionally, the application of ADC technology in non-oncology diseases represents a new and promising frontier yet to be fully explored.
These untapped blue-ocean markets, combined with clinically validated efficacy in certain disease areas and an ever-expanding therapeutic scope, are driving exponential growth in the commercial potential of ADCs. The market prospects are being re-evaluated by the industry. Within this landscape, innovative forces, particularly from China, are on the rise. According to Beacon Reports, as of November 28, 2025, China has become a hotbed for ADC innovation, accounting for 28% of global ADC assets. Significantly, China has emerged as the de facto leader in ADC clinical trials: since 2023, over 50% of global clinical trials and 75% of Phase III combination trials have been conducted in China. Over the past decade, the potential deal value in China's domestic ADC market has surged to US$ 24 billion, with an annual growth rate of 18%.
It is evident that China's ADC sector has evolved from "single-target, single-molecule" follow-on innovation to a global hub for licensing deals, characterized by "platform-level output" capabilities. Moreover, MediLink's sustained recognition underscores the prevailing value assessment paradigm in the current ADC arena: the pinnacle of a biotech company's development is not defined by the peak sales of a single product, but rather by its ability to transform technology into a platform capable of sustainable output and premium valuation. In recent years, MediLink's successful consecutive out-licensing deals for molecules targeting B7-H3, c-MET, HER3, and other targets, culminating in a major transaction centered on its technology platform itself, demonstrates its transition from realizing value in individual projects to gaining validation from top-tier global partners for its platform capabilities.
