Sanofi doubles down on China‘s Helixon in second AI bispecific deal worth up to $2.56B+
On January 5, 2026, Earendil Labs, an overseas affiliate of Helixon, announced a new collaboration agreement with the multinational pharmaceutical company Sanofi. The partnership focuses on an AI-driven drug discovery platform to advance multiple projects in autoimmune and inflammatory diseases.
According to publicly disclosed information, Sanofi will be responsible for the global clinical development and commercialization of bispecific antibody candidates generated through this collaboration, while Earendil Labs will receive a $160 million upfront payment plus near-term milestones tied to early-stage project progress. If all predefined milestones across subsequent R&D and commercialization stages are achieved, the total potential deal value could reach up to $2.56 billion. Additionally, Earendil Labs will be eligible for tiered royalty payments in the high single- to double-digit percentage range based on future product sales.
This is not the first collaboration between the two companies. Looking back to April 17, 2025, Sanofi had previously secured global exclusive rights to two bispecific antibodies from Helixon—HXN-1002 (targeting α4β7/TL1A) and HXN-1003 (targeting TL1A/IL-23)—with an upfront payment of $125 million and a total potential deal value of up to $1.845 billion.
Helixon: AI Accelerates Bispecific Antibody Development, Secures Over $4.4 Billion Collaboration with Sanofi in 2 Years
The core reason Helixon has drawn industry attention by securing two licensing deals with Sanofi in under a year lies not in any single candidate drug, but in its mature antibody discovery and optimization platform capabilities. Helixon was incubated by the Institute for AI Industry Research, Tsinghua University in 2021 and founded by computational biology expert Jian Peng. From its inception, Helixon has established itself as a platform company focused on AI-driven antibody drug discovery.
Technologically, Helixon focuses on the field of bispecific antibodies. In autoimmune and inflammatory diseases, inhibiting a single signaling pathway often fails to address the complex mechanisms of immune dysregulation, and some patients show limited response to single-target biologics. Dual-target intervention is therefore considered promising for achieving more complete inflammation control. However, bispecific antibody development has long faced challenges such as difficulty in synergistic target design, lengthy screening cycles, and high failure rates, typically requiring 3–5 years from molecular design to preclinical validation.
Helixon aims to compress this timeline by integrating AI with high-throughput experimentation. The core logic is to use deep learning models to predict antibody-antigen interactions before experimentation, identifying potential effective target combinations and antibody sequences. High-throughput technology is then employed to rapidly conduct large-scale characterization tests, followed by multiple rounds of iteration to optimize the molecule's developability and stability. This system does not replace experimentation but reduces ineffective trial-and-error, enhancing early screening efficiency—an approach already validated through its out-licensing projects.
The molecule licensed to Sanofi in 2025, HXN-1002, simultaneously targets the α4β7 integrin and TL1A (TNF-like ligand 1A). The former is involved in immune cell migration to the intestinal mucosa, while the latter regulates intestinal inflammation. In treating ulcerative colitis (UC) and Crohn's disease (CD), the dual-target design aims to improve the stability of inflammation control through synergistic inhibition, with preclinical models already demonstrating mechanistic synergy. Another candidate, HXN-1003, targets both TL1A and IL-23 (Interleukin-23), both of which are involved in the inflammatory cascade of autoimmune diseases. This combination design strengthens overall intervention in the inflammatory network while avoiding the uncertainties associated with entirely novel targets. Both molecules are currently in early-stage development, with their value concentrated in the logic of target combination and the methodology of molecular discovery.
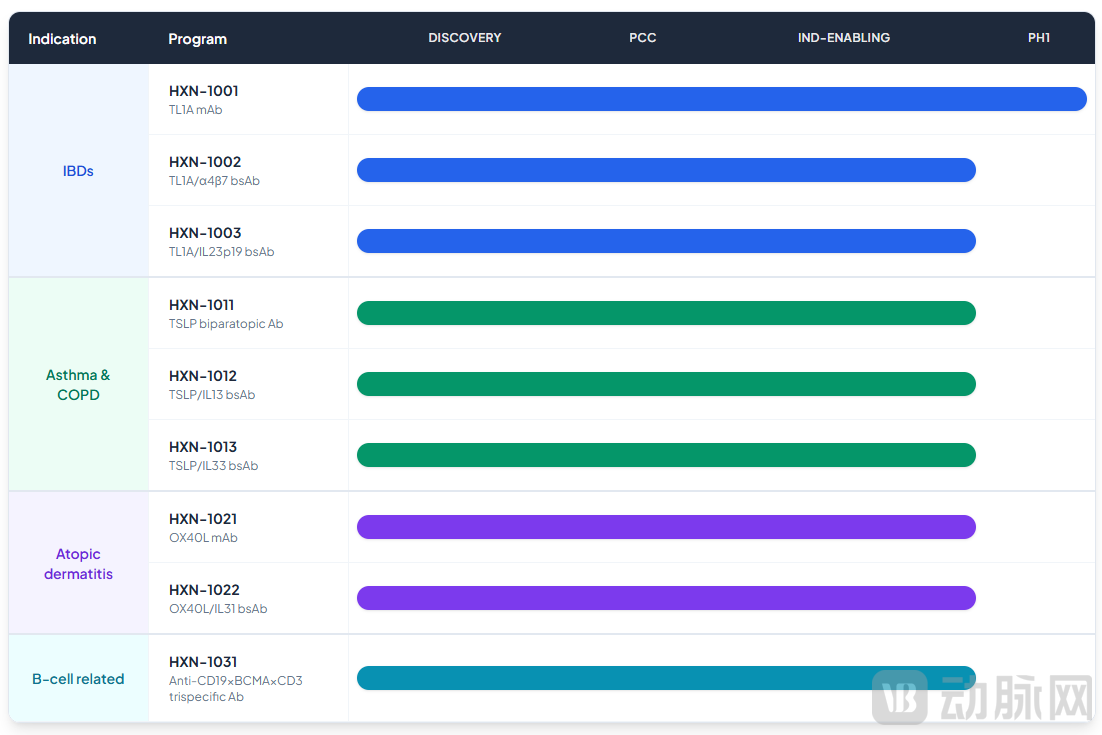
Earendil Labs: Pipeline Overview in Immunology and Inflammation
To summarize, Helixon has entered the global stage not by relying on a single project, but through its AI-driven antibody discovery platform, which offers a more efficient early-stage R&D pathway in the specialized field of bispecific antibodies. This capability forms the core foundation for its sustained collaboration with the same multinational pharmaceutical company within a short timeframe.
Sanofi: €13 Billion Blockbuster Sustains Autoimmune Business, External Collaborations Strengthen Bispecific Antibody Pipeline
From Sanofi's perspective, its two collaborations with Helixon represent strategic steps to strengthen and diversify its autoimmune disease pipeline. Founded in 1973 and headquartered in Paris, France, Sanofi operates across prescription medicines, vaccines, and consumer healthcare. In 2024, Sanofi reported sales revenue of €41.081 billion (an 11.3% year-on-year increase at constant exchange rates). Within this total, its core autoimmune product dupilumab (brand name Dupixent®) achieved single-product sales of €13.072 billion, establishing itself as one of Sanofi's most significant revenue pillars.
In recent years, Sanofi has been steadily sharpening its R&D focus. Since 2019, Sanofi has gradually scaled back innovation efforts in areas such as diabetes and cardiovascular diseases, reallocating resources to priority fields including autoimmune disorders, oncology, and vaccines. In the autoimmune space, despite having established products like dupilumab, existing single-target biologics still face challenges such as insufficient efficacy and drug resistance in indications like inflammatory bowel disease (IBD) and psoriasis. Against this backdrop, Sanofi has turned to external collaborations to bring in novel molecular formats, such as bispecific antibodies, to complement its internal pipeline.
Market Outlook: With 40% CAGR, AI-Powered Bispecific Antibodies Become a New Frontier, Redefining Partnerships
The value of artificial intelligence in drug discovery is evolving from an efficiency tool into a form of systematic innovation capability that multinational pharmaceutical companies are willing to acquire. The field of complex molecular entities, exemplified by bispecific antibodies, serves as a prime example of where this transformation is taking practical shape.
Signals of change are already evident at the market level. According to data from Global Industry Analysts, the global bispecific antibody market was valued at approximately $14.8 billion in 2024 and is projected to grow to the $100 billion tier by 2030, with a compound annual growth rate exceeding 40%. Within the autoimmune therapy landscape, traditional pathways such as TNF-α and IL-23 have become highly crowded, and the efficacy ceilings and drug resistance issues associated with them are increasingly apparent. Bispecific antibodies are regarded as a next-generation solution, yet their engineering complexity and high screening costs remain significant barriers. The integration of AI has markedly compressed the early-stage R&D timeline for bispecific antibodies—a key reason why multinational pharmaceutical firms are now willing to lock in assets at an earlier stage.
Helixon is not an isolated case. In recent years, several Chinese biotech companies have incorporated AI capabilities into the design and optimization processes for bispecific antibodies. WuXi Biologics, leveraging its in-house generative AI platform WuXi ProDesign, generated 30 candidate molecules for a PD-L1/CTLA-4 bispecific antibody in just about two months, three of which were validated through wet-lab experiments and some have advanced to the preclinical stage—far exceeding the traditional discovery cycle of six months or more. Xtalpi's AI platform also secured a strategic collaboration with Eli Lilly in 2025 valued at $345 million, focusing on the AI-driven discovery and development of bispecific antibodies.
The synergy between technological breakthroughs and market demand has not only driven profound changes in collaboration models but has also reshaped value structures. In terms of collaboration, compared to earlier licensing deals primarily centered on late-stage clinical pipelines, multinational pharmaceutical companies now increasingly prefer to engage in partnerships at the drug discovery or preclinical stages. Under these agreements, Chinese biotech firms rapidly generate differentiated lead assets, which are then taken over by multinational corporations for subsequent global development and commercialization. In terms of value recognition, the "China Healthcare Industry White Paper 2025" by CEC Capital indicates that the average value of out-licensing transactions by Chinese biotech companies in 2024 was approximately $420 million. In contrast, multiple recent AI-driven innovation collaborations have seen potential deal sizes surging to over $1 billion—for instance, CSPC's AI drug discovery platform collaboration with AstraZeneca reached up to $5.33 billion, and Harbour BioMed's AI-driven multispecific antibody platform partnership with Bristol Myers Squibb also totaled $1.035 billion.
It is evident that Sanofi's two collaborations with Helixon are not isolated events but rather a microcosm of the global restructuring of the drug R&D chain: technological innovation is shifting earlier, partnerships are deepening, and Chinese biotech companies are moving beyond the "pipeline export" framework to become integral early-stage collaborators in the global R&D ecosystem.
