Dual CE & NMPA approval: Spectrumedics’ Sonico-CX IVL system emerges as a breakthrough solution for severe coronary calcification

Recently, Spectrumedics announced that its independently developed Sonico-CX Coronary Intravascular Lithotripsy (IVL) System (hereinafter referred to as "Sonico-CX") has officially received CE certification in the European Union. The system consists of a single-use coronary intravascular lithotripsy catheter and an IVL generator. It is designed to safely modify severely calcified plaques through shockwave energy, thereby creating ideal conditions for subsequent stent implantation.
Compared with the first-generation products of international industry leaders, Sonico-CX offers four major advantages: a smaller balloon folded outer diameter (0.041 inches, approximately 1.04 mm), a higher number of available pulses (120 pulses), more uniform energy delivery (enabling 360-degree circumferential and intermittent shockwave emission), and a more comprehensive range of catheter specifications (7 sizes ranging from 2.5 mm to 4.0 mm). These features allow the system to precisely match coronary vascular anatomical characteristics, effectively improving balloon wall apposition and plaque compliance, reducing treatment blind spots, and thereby increasing procedural success, reducing complications, and improving patient outcomes.
It is noteworthy that this system received approval from China's National Medical Products Administration (NMPA) for market launch in March 2024 and obtained regulatory approval in Ecuador in November of the same year, making it the first China-developed IVL product to secure overseas certification. Now, one and a half years later, achieving CE certification not only further validates the system's technical safety and clinical efficacy but also confirms that Spectrumedics has established a research & development, quality, and regulatory compliance system in line with international first-class standards. This demonstrates Spectrumedics' capability to consistently deliver high-standard medical devices across different regulatory frameworks.
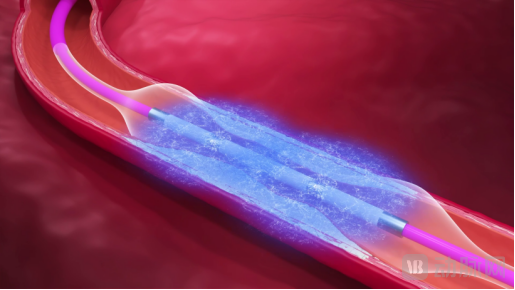
With the increasing aging population and the rising number of patients with metabolic diseases, the incidence of moderate-to-severe coronary calcified lesions in clinical practice has significantly increased. Particularly, complex lesion patterns such as circumferential calcification and deep calcification, due to their significant impact on vessel compliance, have become key factors limiting the success rate and long-term outcomes of percutaneous coronary intervention (PCI).
However, current treatment modalities are gradually approaching their limitations. Passive interventional devices such as cutting balloons and scoring balloons are only suitable for mild to moderate calcification. Rotational atherectomy (RA), although currently a primary treatment for severe calcification, is limited to modifying intimal calcified plaques and cannot address deep, subintimal calcified lesions. It also carries risks of complications such as vessel perforation and myocardial infarction. More critically, its operation is complex with a steep learning curve, making it difficult to adopt in primary care hospitals. This has long posed a clinical trade-off between efficacy and safety.
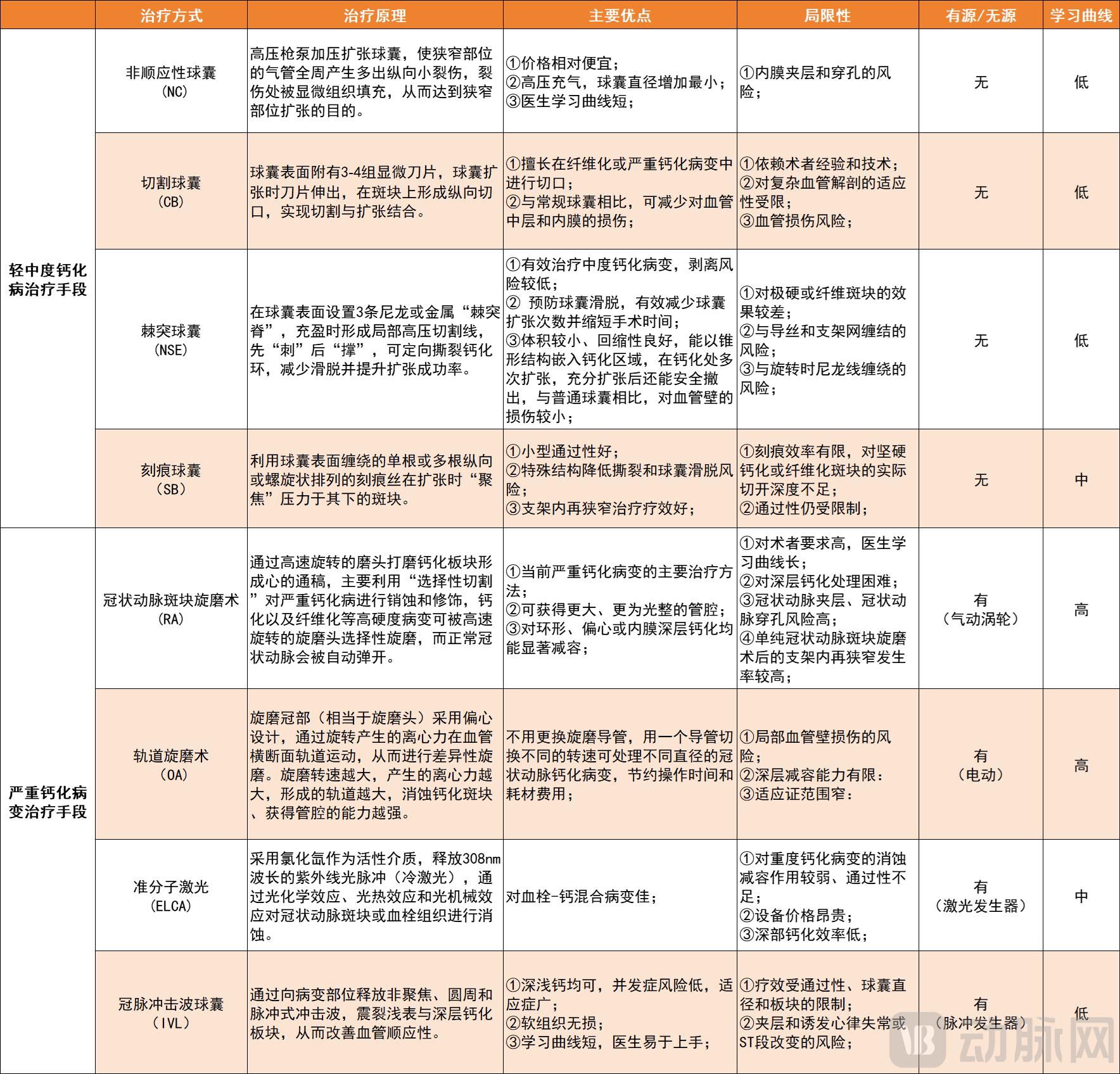
Current Treatment Methods for Calcified Lesions, Chart by VCBeat
Against this backdrop, intravascular lithotripsy (IVL) has gradually emerged as a key breakthrough direction in the interventional treatment of calcified lesions. The technology utilizes a balloon to deliver pulsed shockwaves, which can penetrate the intima to reach deep calcifications, achieving fracture of calcified plaques without damaging the vessel wall. This safely improves vessel compliance and is characterized by minimal trauma, ease of operation, a gentle learning curve, and broad indications. It not only helps to simplify procedural steps and shorten procedure time but also reduces related complications and the need for subsequent treatments, thereby lowering overall procedural risk to a certain extent. IVL provides a new therapeutic pathway for the interventional management of complex calcified lesions.
Thanks to these advantages, since 2018, IVL has been successively approved for market launch in Europe, the United States, and China, and has been recommended by multiple clinical guidelines and expert consensus documents. The Chinese Expert Consensus on the Diagnosis and Treatment of Coronary Artery Calcified Lesions (2021 Edition) stated that it described it as a potential "game-changer" for coronary artery calcified lesions. Furthermore, the Clinical Application of Percutaneous Coronary Intraluminal Shock Wave Balloon Catheter Angioplasty: Recommendations from Chinese Experts (2023) further clarified that IVL is suitable for various types of severe calcified lesions, including concentric (or circumferential) and eccentric calcifications, as well as focal and diffuse calcifications.
360° Circumferential Delivery, 120 High-Energy Pulses, Enhanced Deliverability: Sonico-CX Enables Precise Calcification Modification
Sonico-CX System (Subject to the final product)
While IVL technology is advancing toward guideline recommendations, clinical practice still holds higher expectations for uniform energy coverage, adequate capacity to handle long or diffuse calcified lesions, improved catheter deliverability, and better adaptability to operator preferences.
To address these clinical needs, the Sonico-CX system has incorporated numerous design innovations. It aims to enhance the clinical value and promote the global adoption of IVL in complex coronary calcified lesions by delivering more complete energy release, greater procedural flexibility, and superior vessel access.
By enabling 360° circumferential and uniform shockwave emission through targeted innovations in its core structure, Sonico-CX achieves more complete energy delivery. This allows energy to be applied to the calcified plaque with greater precision and stability, reduces energy blind spots during the procedure, and thereby enhances the completeness of treatment for complex calcified lesions.
The Sonico-CX system flexibly addresses complex multi-lesion scenarios by combining 120 high-energy pulses with a diverse range of catheter sizes. Compared to the 80-pulse limitation common to most IVL products, a single Sonico-CX catheter supports up to 120 pulse deliveries, providing ample procedural margin for treating long lesions, diffuse calcification, and other challenging cases. Additionally, the system offers seven different sizes of lithotripsy catheters, achieving refined and precise coverage of coronary arteries with varying diameters.
The system significantly improves lesion access capability by reducing the crossing profile and enhancing deliverability. In direct response to clinical feedback regarding the insufficient deliverability of imported products, Spectrumedics has substantially minimized the catheter's crossing profile and improved its trackability, facilitating easier navigation through tortuous vessels. The balloon catheter of the second-generation product (in development) will feature a notably smaller profile compared to the second-generation offerings from international industry leaders, granting the system superior access capability in tortuous and angulated lesions.
The dual trigger mode, incorporating both "foot pedal" and "handle button" options, aligns with the operational habits of a global user base. Acknowledging the differences in operating preferences and energy delivery habits among international operators, Spectrumedics has undertaken targeted optimizations in the energy triggering mechanism and user interface logic. Unlike the single-handle-button design of market leaders, which presents limitations in single-operator scenarios, Sonico-CX offers a dual trigger system: the foot-pedal design is optimized for independent operator settings, freeing both hands to enable solo operation, while the handle-button trigger maintains compatibility with traditional surgical modes. These two operating modes provide flexible choices tailored to operator preference.
These system-level optimizations, centered on enhancing the operator experience and driven by fundamental technological innovation, not only improve the therapeutic outcomes of IVL technology in complex lesions but also lower the procedural learning curve. This establishes a solid foundation for the standardized promotion and global adoption of the Sonico-CX system.
The European Union's CE Marking certification, particularly for innovative energy-based interventional devices, is widely regarded as one of the most stringent thresholds in the global regulatory system. The successful acquisition of the CE Mark for Sonico-CX not only marks a significant technological breakthrough for Spectrumedics in the field of interventional medicine but also reflects the company's deep-rooted capabilities in clinical research, quality control, and corporate governance systems. This achievement demonstrates that Spectrumedics possesses the systematic capability to consistently produce high-quality innovative products capable of meeting the exacting standards of the world's most demanding markets.
In fact, prior to entering the European market, Spectrumedics had already been actively advancing the clinical application of IVL technology in over ten countries across Asia, Latin America, and the Middle East. Through real-world clinical practice at leading hospitals in these regions, the company has progressively accomplished the transition from mere "product entry" to gaining "clinical acceptance," thereby establishing a preliminary foundation of international trust for the China-developed IVL system.
Building upon this foundation, Spectrumedics' internationalization strategy is evolving from focusing on single-market expansion to fostering the systematic promotion and collaborative capability-building for IVL technology itself. In September 2025, Spectrumedics and the Xiamen Cardiovascular Hospital Xiamen University jointly established an IVL Joint Training Center specifically for international academic exchange. This center focuses on standardizing IVL operation protocols, clinical validation, and professional training, creating a platform for technology incubation and knowledge transfer geared toward the international market. Leveraging this center, both parties have initiated multi-level academic exchanges and clinical training programs in several countries participating in the Belt and Road Initiative, including Thailand, Indonesia, Vietnam, and Pakistan, to promote the standardized adoption of IVL technology across diverse healthcare systems.

On the left is Professor Wang Yan, Dean of the Xiamen Cardiovascular Hospital Xiamen University, and on the right is Dr. Peng Huiqun, Founder and CEO of Spectrumedics
From a longer-term perspective, this model not only helps improve the accessibility of Chinese innovative medical devices overseas but also actively contributes to the co-creation of clinical pathways and application standards for IVL technology. For Spectrumedics, its role is evolving from being solely a product provider to becoming a promoter of IVL technology and an exporter of clinical methodology.
The CE Marking represents a crucial milestone in Spectrumedics' internationalization strategy and, more importantly, serves as the starting point for a new phase of its global journey. Seizing this opportunity, Spectrumedics will fully initiate its commercial rollout in Europe and progressively enter other high-end markets, such as North America. In the future, Spectrumedics will continue to expand the application boundaries of IVL technology, extending its use from coronary interventions to broader clinical scenarios including cardiac valve calcification and peripheral vascular interventions, aiming to deliver safer, more efficient, and more precise minimally invasive interventional treatment options for patients worldwide.
