A mother-son led biotech: B&K Corporation’s HKEX listing to fuel its PDGF ambitions
On December 22, B&K Corporation successfully listed on the Hong Kong Stock Exchange under the stock code 2396.HK. The joint sponsors for the listing were Huatai Securities and CITIC Securities.
Founded in 2012, B&K Corporation is the first innovative biopharmaceutical company listed in Hong Kong with a core focus on platelet-derived growth factor (PDGF) drugs. Currently, the global PDGF drug market has only one approved product, Regranex, which received FDA approval in the United States. In China, no PDGF drugs have been commercialized yet, making this therapeutic area a blue-ocean market. This promising sector has also garnered significant capital support. From 2021 to 2023, B&K Corporation completed three financing rounds, raising a total of RMB 405 million. Investors included Qingdao Gaoke Communication and CDH Investment, among others. Following its Series B financing round in 2023, the company's post-money valuation reached RMB 3.3 billion.
For this IPO, B&K Corporation opened at HKD 33.8 per share. As of 10:00 AM, its total market capitalization was approximately HKD 4.083 billion. According to its prospectus, approximately 61.8% of the net proceeds from the offering will be allocated to the continued clinical development and commercialization of its core products—Pro-101-1 (for the treatment of burns) and Pro-101-2 (for the treatment of diabetic foot ulcers, DFUs)—which is the primary reason for the listing. The remaining funds will be used for the procurement of specialized equipment and instruments related to R&D and quality control activities, preclinical research for other PDGF products and non-PDGF drug candidates, as well as general corporate purposes.
A Mother-Son Duo at the Helm of B&K Corporation
The history of B&K Corporation can be traced back to 2012, when its predecessor, Beijing Zhonghong Saisi Biotechnology, was established. The company was co-founded by Jia Lijia—the founder, Chairman of the Board, Executive Director, and one of the controlling shareholders of B&K Corporation—alongside Li Gewei, another controlling shareholder of B&K Corporation, and two other minority shareholders at the time. In 2020, Zhonghong Saisi was renamed Beijing B&K Corporation. In 2023, it underwent a further name change to B&K Corporation, and simultaneously relocated its registered office address from Beijing to Qingdao.
Since its inception, B&K Corporation has consistently focused on discovering, developing, and commercializing therapies for wound healing, particularly platelet-derived growth factor (PDGF) drugs. PDGF is one of the growth factors secreted by platelets after injury, which promotes (among other functions) the formation of new blood vessels, modulates inflammation, and stimulates cell proliferation and migration, ultimately accelerating wound healing. According to a Frost & Sullivan report, the market size for wound healing drugs in China increased from RMB 82.8 billion in 2018 to RMB 95.7 billion in 2024 and is projected to reach RMB 118.0 billion by 2033.
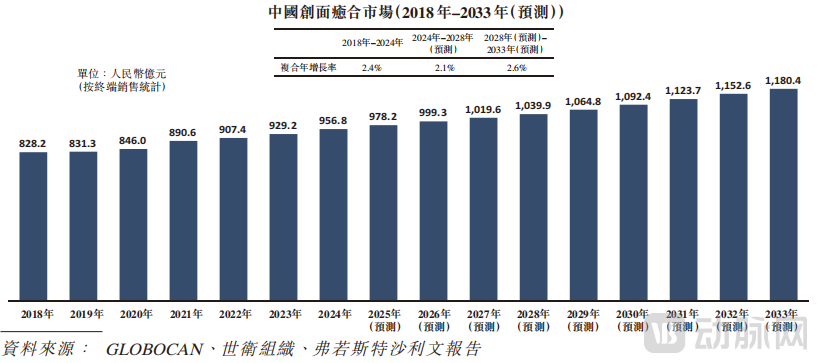
Figure 1. China's Wound Healing Market, Source: Prospectus
Currently, PDGF is the only recombinant growth factor approved by the FDA for topical use, with a clinical history of over 20 years in treating diabetic foot ulcers. However, the market for this drug is primarily concentrated in the United States, while no commercialized PDGF drugs are available for this application in China, indicating significant future market potential.
In addition to the indications B&K Corporation focuses on, such as wound healing, pressure ulcers, radiation-induced ulcers, dry eye disease, corneal injury, solar dermatitis, hair loss, hemorrhoids, and gastric ulcers, a Frost & Sullivan report suggests that PDGF drugs hold promise for nearly 20 other indications across various medical specialties. These include general surgery (e.g., varicose vein ulcers, phlebitis, and lower limb venous ulcers), radiotherapy (e.g., skin repair post-radiation), dermatology, medical aesthetics (e.g., wound care after plastic surgery), ophthalmology (e.g., keratitis, refractive surgery, refractive errors, cataracts, and glaucoma), orthopedics (e.g., tennis elbow, fasciitis, osteoarthritis, and osteoporosis), dentistry (e.g., gingival recession, periodontal disease, and alveolar bone defects), and obstetrics and gynecology (e.g., cesarean section wound care), among others.
B&K Corporation's precise focus on this blue-ocean market is closely tied to the leadership behind the company. Its founder, Jia Lijia, has served as a Director and Chairperson of the Board since the company's establishment in April 2012 and was redesignated as an Executive Director in April 2024. She is primarily responsible for leading and governing the Board and overseeing the Group's overall business strategy and management.
Jia Lijia possesses over 27 years of experience in the pharmaceutical industry. Prior to founding B&K Corporation, she served as Sales Manager at Mudanjiang Lingtai Pharmaceutical (Beijing Office) from January 1997 to September 2004. Subsequently, from October 2004 to December 2010, she held the position of Deputy General Manager at Beijing Shenghongye Pharmaceutical, a company primarily engaged in pharmaceutical technology development, where she was mainly responsible for sales and operational management.
Unlike most founders of innovative drug companies who come from a research background, Jia Lijia's expertise lies in pharmaceutical sales and operations. Additionally, another key executive at B&K Corporation, Wang Kelong—who is also Jia Lijia's son—does not have a formal medical or pharmaceutical education background. Serving as the Vice Chairperson of the Board and President of the Company, Wang Kelong has been a Director since October 2020 and was redesignated as an Executive Director in April 2024. He is primarily responsible for supervising the execution of B&K Corporation's overall strategy, business development, management, and financing.
Wang Kelong brings nine years of corporate operations and management experience. Before joining the Group, he worked at Berkshire Hathaway Automotive, one of the largest automotive retail groups in the United States. Later, he founded Beijing Luqi Technology, where he served as its Chief Executive Officer from April 2017 to September 2020, overseeing Beijing Luqi Technology's overall operations.
Although Wang Kelong does not come from a pharmaceutical background, his prior achievements in other fields have attracted considerable attention. He has been recognized on the 2019 Forbes China 30 Under 30 list, the 2024 Fortune China 40 Under 40 list of promising business elites, the 2018 Hurun 30×30 Young Entrepreneurs list, the 2024 Hurun China Healthcare Young Entrepreneurs list, and the 2025 Fortune China 40 Under 40 list. Furthermore, he is a co-author of several research papers in the fields of network intelligence and drug delivery.
Within the pharmaceutical industry, the mother-son leadership model is relatively uncommon, which may attract investor attention while also potentially sparking discussions about B&K Corporation's governance structure and professional expertise. Nevertheless, Jia Lijia's extensive pharmaceutical experience and Wang Kelong's profile as a young elite have indeed shaped a distinctive market image for B&K Corporation.
Establish a Protein/Peptide and Nucleic Acid Platform, with Core Personnel Having Led SARS and H1N1 Projects
Although some core members do not come from a traditional pharmaceutical background, the scientific research capabilities of B&K Corporation are formidable. The company has established two core technology platforms: a Protein/Peptide Therapeutics Platform and a Nucleic Acid Therapeutics Platform.
Protein/Peptide Therapeutics Platform: This platform provides B&K Corporation with the capability for novel drug formulation development and indication expansion. It plays a pivotal role in the development of the company's pipeline, particularly in advancing PDGF (Platelet-Derived Growth Factor) therapies. The platform incorporates both eukaryotic and prokaryotic expression technologies. Firstly, the eukaryotic expression system based on Pichia pastoris is critical for ensuring the quality and yield of PDGF products. Secondly, the prokaryotic expression system utilizes Escherichia coli, offering advantages such as simple culture conditions, rapid growth and reproduction, high safety, cost-effectiveness, high efficiency, and strong scalability. These characteristics make it an ideal expression system for producing recombinant proteins and peptides, and it is expected to enrich B&K Corporation's pipeline of protein/peptide therapeutics based on this system.
Nucleic Acid Therapeutics Platform: This platform is built upon mRNA molecular design and Lipid Nanoparticle (LNP) delivery technologies. It enables the development of mRNA and ASO (Antisense Oligonucleotide) candidates targeting indications such as solid tumors, gliomas, and TNBC (Triple-Negative Breast Cancer). The mRNA molecular design technology helps ensure high-level expression of mRNA therapeutics while minimizing potential side effects. Concurrently, the LNP delivery technology assists B&K Corporation in designing and screening ionizable lipids to identify proprietary candidate molecules. Furthermore, the company intends to further develop a biomolecular therapeutic development platform to support broader application scenarios for its candidate products.
Supporting the core leadership is a professional R&D team with extensive drug development experience.
For example, Zhai Junhui, General Manager of B&K Corporation, possesses approximately 30 years of experience in biomedical scientific research. His primary research areas include microbiology and viral genomics, the discovery of new pathogens in emerging infectious diseases, and the development of novel viral detection technologies. He holds a Ph.D. in Preventive Health Sciences from the Academy of Military Sciences and served as a postdoctoral researcher in microbiology at the Columbia University Mailman School of Public Health (Center for Infection and Immunity Laboratory), under the mentorship of the renowned biomedical expert and "virus hunter," Professor Walter Ian Lipkin.
Zhai Junhui previously served as a researcher at an institute of the Academy of Military Sciences, where he led and participated in several national and other major medical projects, such as the development of nucleic acid-based in vitro diagnostic reagents for SARS and H1N1 vaccines. He also served as a United Nations Biological Weapons Inspector for Iraq and was the Deputy Head of the Biosafety Team for the 2008 Beijing Olympics. He has published over 100 scientific papers on microbiology, viral genomics, and novel viral detection technologies and holds multiple national invention patents. Additionally, to date, Zhai Junhui is a co-inventor on 27 of B&K Corporation's patent applications, 10 of which have been granted.
Zhao Xinghui, Chief R&D Officer of B&K Corporation, brings about 20 years of experience in biomedical scientific research. Her primary research focuses include protein-engineered drugs, mechanisms of pathogen infection, tumor molecular markers, epigenetic regulation, and hematopoietic stem cell aging. She is proficient in various expression systems, including those based on E. coli, P. pastoris, and CHO mammalian cells. To date, she has published 37 SCI-indexed papers, which have been cited approximately 1,000 times, resulting in an H-index of 19. Furthermore, to date, Zhao Xinghui is a co-inventor on 35 of B&K Corporation's patent applications, 7 of which have been granted.
Backed by this R&D team and a robust patent portfolio, B&K Corporation has established a pipeline of 10 candidate products based on its Protein/Peptide and Nucleic Acid Therapeutics Platforms, encompassing 14 indications. These include a PDGF pipeline and an mRNA/ASO pipeline. Seven of these candidates are PDGF-based drugs intended for a wide range of wound healing indications, including burns, diabetic foot ulcers, fresh wounds, pressure ulcers, radiation-induced ulcers, dry eye disease, corneal injury, solar dermatitis, hair loss, hemorrhoids, and gastric ulcers. They feature diverse formulations such as topical gels, sprays, eye drops, oral preparations, and device-supported delivery routes.
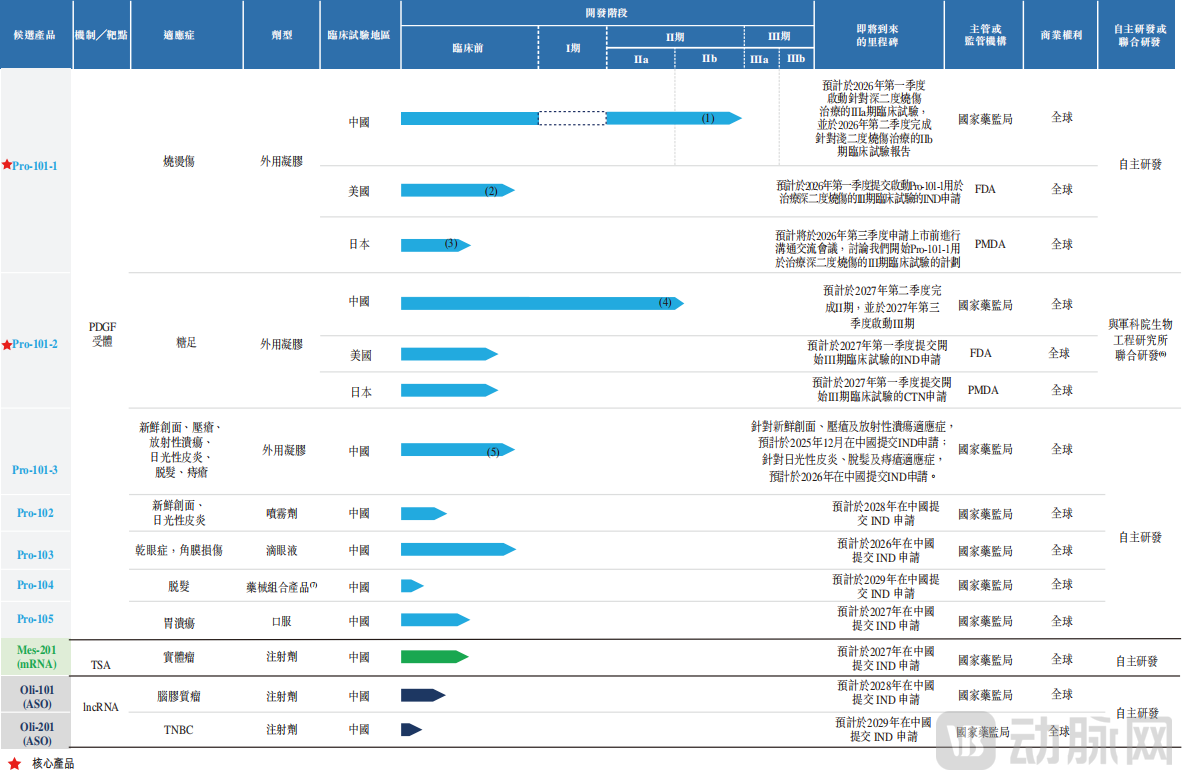 Figure 2.Product Pipeline of B&K Corporation, Source: Prospectus
Figure 2.Product Pipeline of B&K Corporation, Source: Prospectus
Only Two Pipelines Approved for Clinical Trials, with a Loss of 450 Million in the Past Three Years
Currently, B&K Corporation has two core products. These include Pro-101-1 for the treatment of deep second-degree burns, for which the statistical data analysis of the Phase IIb clinical trial has been completed, and for the treatment of superficial second-degree burns, for which the last patient has completed the trial (last-patient-out), though statistical results are pending. The other core product is Pro-101-2 for the treatment of diabetic foot ulcers (DFU), which is currently in the Phase II clinical trial stage.
According to a Frost & Sullivan report, Pro-101-1 is the PDGF candidate drug with the most advanced clinical development for treating burns in China. For deep second-degree burns, Pro-101-1 has completed the statistical data analysis of its Phase IIb clinical trial, with plans to initiate a Phase IIIa trial in the first quarter of 2026 and complete it in the second quarter of 2026. For superficial second-degree burns, Pro-101-1 has reached last-patient-out, and data statistical analysis is expected to be completed in the first quarter of 2026. The decision to proceed to a Phase III trial will depend on the Phase IIb results and subsequent communication with the Center for Drug Evaluation (CDE).
Regarding commercialization planning, B&K Corporation has initiated preparations for the Phase III clinical trial of Pro-101-1 and plans to submit a New Drug Application (NDA) in 2026. For market expansion, B&K Corporation has formed a composite commercialization team covering areas such as wound care and medical aesthetics and has established a clinical research collaboration network with several top-tier hospitals.
B&K Corporation also plans to launch Pro-101-1 in the United States while establishing its foothold in the Chinese market, marking the beginning of its internationalization strategy. In December 2021, B&K Corporation submitted a pre-IND communication request to the FDA for Pro-101-1 for burn treatment. The FDA agreed with B&K Corporation's proposal to conduct clinical trials and to submit a Biologics License Application (BLA) for Pro-101-1 for burn treatment via an innovative biologics approval pathway.
B&K Corporation's other core pipeline asset, Pro-101-2, was initially co-developed with the Academy of Military Sciences (AMS). In July 2021, when the IND for Pro-101-2 was approved, AMS remained a joint sponsor. However, since the IND approval, AMS has not participated in its clinical studies or related drug development work. Therefore, the prospectus indicates that upon completion of clinical development, B&K Corporation is expected to be the sole holder of the Marketing Authorization Holder (MAH) license for Pro-101-2. Furthermore, AMS has transferred the project-related technical materials to B&K Corporation, and the latter holds exclusive use and commercialization rights for two PDGF-related patents. Consequently, AMS is not authorized to license the project-related technical materials or PDGF-related patents to any third party without B&K Corporation's consent.
Currently, Pro-101-2 is in the Phase II clinical trial stage. It is expected to complete Phase II in the second quarter of 2027 and initiate a Phase III trial in the third quarter of 2027. The company plans to conduct international multi-center clinical trials simultaneously in the United States and Japan to accelerate the global commercialization process.
Apart from Pro-101-1 and Pro-101-2, B&K Corporation's remaining eight pipeline candidates are all in the preclinical stage. Therefore, B&K Corporation remains in a phase of clinical advancement and R&D investment and has not yet generated stable product commercialization revenue. It is anticipated that substantial R&D investment will continue for a considerable period.
The prospectus shows that B&K Corporation reported net losses of approximately RMB 105 million, RMB 212 million, and RMB 134 million for the years 2023, 2024, and the first nine months of 2025, respectively (aggregating to approximately RMB 450 million). The majority of these losses were attributable to R&D expenses and administrative costs. As of September 30, 2025, B&K Corporation's cash and cash equivalents balance was RMB 73.79 million.
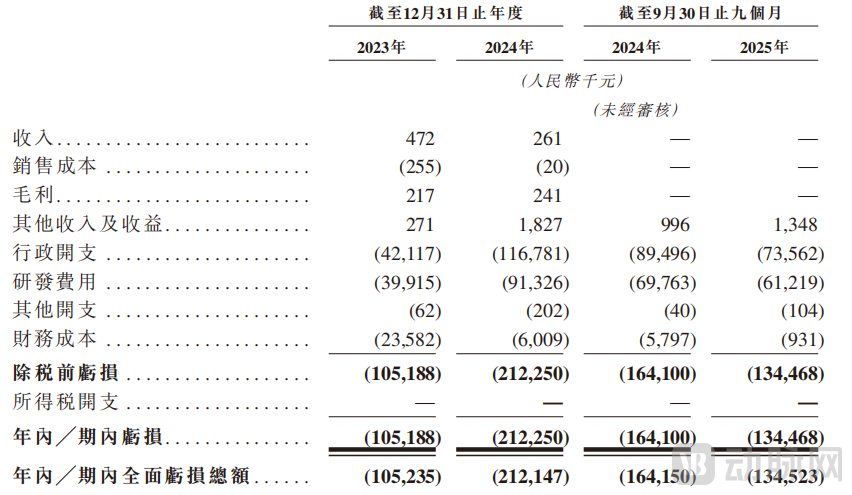
Figure 3. Financial Data of B&K Corporation, Source: Prospectus
It is foreseeable that as its pipeline advances, with its technology platforms validated and key Phase II data locked, B&K Corporation must now prove itself in the real world and on the reimbursement front. Although wound care drugs may not be as high-profile as oncology therapies, they represent a critical entry point for chronic disease management. Any advancement that reduces healing time by even a single day translates directly into lower healthcare costs. Following the bell-ringing ceremony of its listing, it is hoped that with the support of the international capital platform, B&K Corporation will soon fill the PDGF gap in China's wound repair sector, completing a crucial piece of the puzzle.
