JunFuture secures over RMB 10M in angel+ round to scale fluorocarbon production for eye care and blood substitutes
JunFuture (Jiangsu) Biomedicine Co., Ltd. (“JunFuture”) recently completed an Angel+ financing round raising over RMB 10 million. The proceeds will be primarily allocated to the construction of a fluorocarbon active pharmaceutical ingredient (API) production facility at the company's subsidiary in Shandong Province.
Founded in 2021, JunFuture is currently focused on building four innovative drug platforms based on fluorocarbon medicine—injectable, ophthalmic, topical, and inhalable—along with establishing a scaled supply chain for the upstream fluorocarbon raw materials. Leveraging the unique inertness and oxygen-carrying properties of fluorocarbons, these medical products have broad potential applications in treating hypoxia, oxidative stress-related injuries and inflammations, as well as in the temporary filling, protection, and lubrication of organs and tissues.
As an emerging aqueous-free ophthalmic formulation, perfluorohexyloctane and perfluorobutylpentane (0.1% cyclosporine) eye drops have been approved for marketing in China, the United States, Europe, and other regions for the treatment of dry eye disease. They were included as recommended therapies in the American Academy of Ophthalmology's Dry Eye Syndrome Preferred Practice Pattern (PPP) in 2023 and the Chinese Expert Consensus on the Clinical Diagnosis and Management of Dry Eye in 2024, respectively, highlighting their significant market potential.
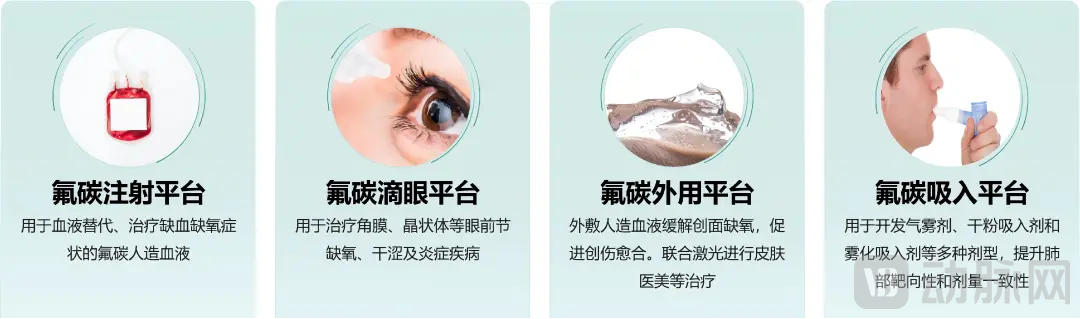
Four innovative drug platforms based on fluorocarbon medicine
Fluorocarbon oxygen carriers hold a unique and irreplaceable advantage in fields such as blood substitution and oxygen-delivery therapy. Notably, Flusol-DA (developed by the Japanese Green Cross Corporation) remains the only artificial blood substitute ever approved for marketing by the U.S. FDA.
As a nation facing significant blood shortages, China reportedly has an annual supply gap of approximately 20 million units of blood. The corresponding market size for blood substitutes is estimated to be close to RMB 50 billion.
In 2022, China's National Medical Products Administration (NMPA) explicitly stated in Document No. 14 that the development of safe and effective blood substitutes is of great significance for emergency treatment under conditions of blood shortage and that such products may be eligible for expedited review pathways.
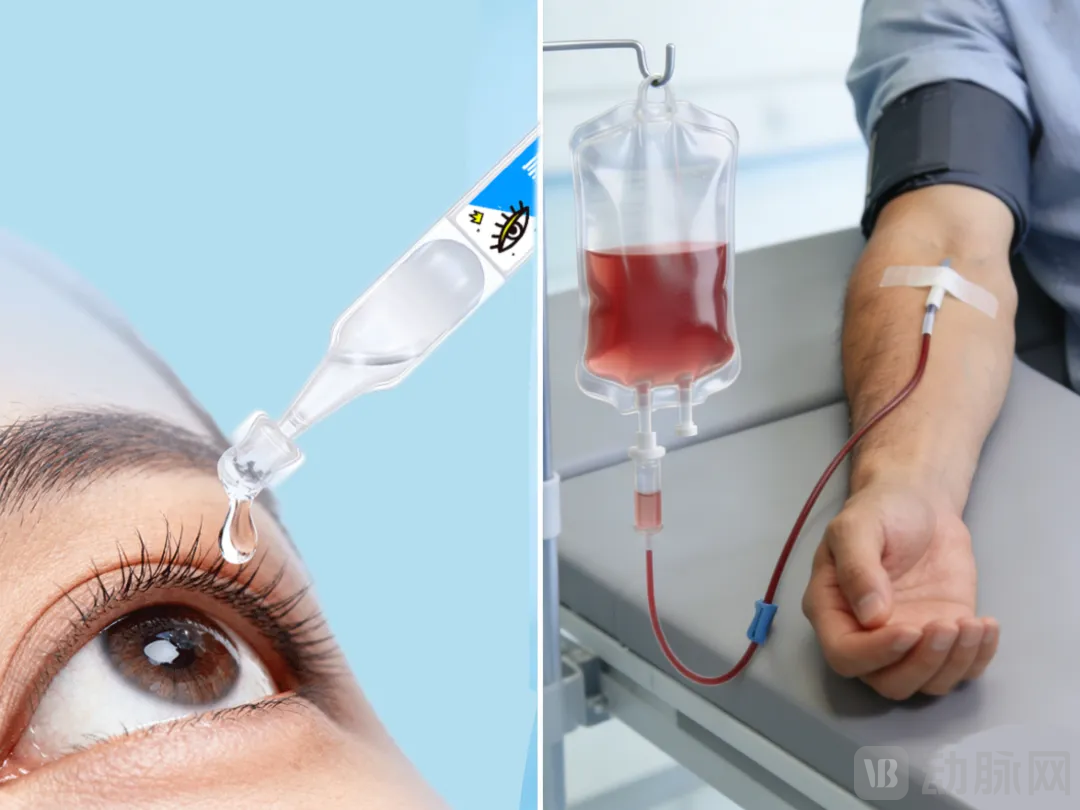
The proceeds from this financing round will accelerate the completion and operational launch of multiple fluorocarbon raw material production lines at JunFuture. These lines are dedicated to manufacturing key compounds, including perfluorobromooctane, perfluorohexyloctane, and perfluorobutylpentane.
This expansion aims to swiftly address the growing domestic and international R&D and supply demands for related raw materials, as well as for finished products such as ophthalmic solutions and artificial blood substitutes.
