AI drug developer DIP raises ~$50M Series D to advance autonomous clinical system and global reach
Global AI-powered drug development leader Deep Intelligent Pharma (DIP) has announced the successful completion of its Series D funding round, raising nearly USD 50 million. The round was led by CDH BAIFU, with continued investment from existing shareholders Xinding Capital and HSG (formerly known as Sequoia Capital China). Index Capital served as the exclusive financial advisor.
The fresh capital will be primarily allocated to the R&D iteration of its end-to-end autonomous clinical system and the expansion of its global commercial network. This investment aims to accelerate DIP's strategic transition from an "assistive tool" to a foundational "decision-making infrastructure" for the industry.
1Defining the "AI Brain" for the Pharmaceutical Industry
Traditional drug R&D faces a "double dilemma": not only are timelines long and costs high, but nearly 40% of failures stem from flaws in clinical trial design or non-scientific factors during execution. The industry's urgent need is no longer simple "labor outsourcing," but rather an "intelligent decision-making system" capable of precisely mitigating risks.

The Dilemma of Clinical Success Rates in Traditional New Drug Development
DIP has broken the mold of the traditional CRO "resource-staffing" service model, redefining the value proposition of AI in the pharmaceutical field—it's not just about "doing it fast," but fundamentally about "doing it right."
"We define success not merely as 'completion,' but as 'achievement,'" said Ms. Xing Li, Founder and CEO of DIP. "Through AI intervention, we conduct comprehensive 'commercial endpoint' simulations from the earliest clinical stages, filtering out execution noise and design flaws. Our commitment is to elevate the asset success rate in drug R&D toward its theoretical limit."
2Fortifying the Technical Moats
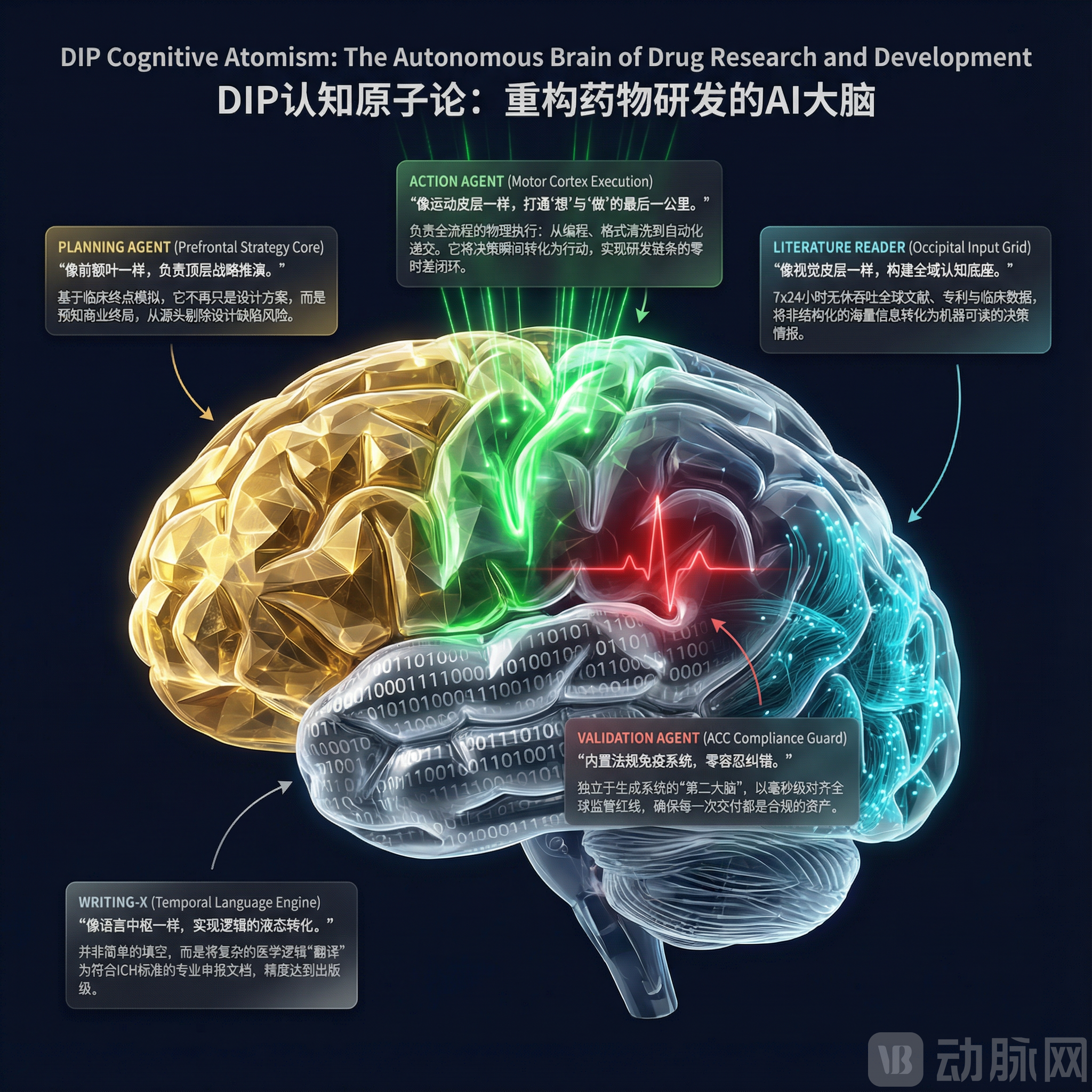
After years of dedicated development, DIP has built the industry's first "Synaptic Agent Ecosystem" based on bionic principles. This "human brain-like" multi-agent ecosystem features three distinct characteristics and advantages:
Cognitive Atomism: DIP deconstructs complex clinical and medical logic into over 10,000 high-precision, specialized "Atomic Agents." From "eligibility criterion validation" to "statistical endpoint simulation," each Agent functions like a top-tier specialist, operating with dedicated expertise while maintaining seamless collaboration.
Bionic Brain with Synaptic Orchestration and Self-Evolution: Leveraging iterative learning from "Dark Data" (Process Knowledge) accumulated across tens of thousands of past projects, DIP's system possesses a "code-level reflection mechanism." When encountering novel development pipelines, the system can formulate hypotheses, conduct validation, and ensure logical consistency akin to a human expert, moving far beyond simple database lookups.
Ultimate Precision, Inheriting Medicine's "Zero-Tolerance" DNA: Imbued with the "zero-tolerance" training ethos inherent to the medical field, DIP's AI deliverables achieve 99.9% industrial-grade precision. This directly addresses the critical pitfall of general-purpose AI models, which are prone to generating "hallucinations" in high-stakes medical scenarios.
3Steady Commercialization and Market Implementation
DIP has established a comprehensive, end-to-end product portfolio encompassing Protocol Agent (Protocol Design Intelligence), Biostatistics, Pharmacovigilance, and Clinical Operations. This integrated suite forms a closed-loop system from "literature research" to "regulatory submission," demonstrating remarkable "disruptive advantage" in real-world validation.
A case in point is the clinical trial protocol design for Immunorock, a pioneering Japanese oral cancer vaccine innovator based in Kobe. To initiate a Phase I/IIa trial for a novel triple-immunotherapy drug targeting oral cancer, Immunorock needed to rapidly draft and submit the protocol to Japan's PMDA. Under traditional models, protocol design heavily relies on individual expert experience, often leading to implementation challenges due to unforeseen complexities.
DIP not only autonomously authored the complete clinical trial protocol within an exceptionally short timeframe but also leveraged "digital twin" technology to simulate the entire patient journey—from screening to data analysis—prior to actual enrollment. This proactive simulation identified potential logical flaws in the protocol that could have led to high patient dropout rates.
This "simulate first, execute later" approach enabled Immunorock to mitigate potential compliance risks and significantly enhance the trial's probability of success. The protocol achieved "zero revisions" and gained PMDA approval in a single review cycle, saving the team 90% of the timeline without dependence on a traditional CRO.
