Molecular glues: the new frontier where MNCs placed $10B+ wagers in 2025
Historically, target identification has been the cornerstone of small-molecule drug development. However, the human body contains approximately 19,000 proteins, the vast majority of which are considered undruggable targets. According to the "Human Protein Atlas", there are currently 5,068 proteins known to be associated with diseases. Of these, approximately 700 have been utilized in approved small-molecule drugs, about 1,200 are considered potentially druggable, while over 3,000 proteins are classified as "undruggable" targets.
Currently, the market size for inhibitor-based targeted drugs—which address only a small fraction of druggable targets—exceeds one hundred billion USD annually. The potential market would be immeasurable if the remaining 3,000-plus undruggable targets could be leveraged for drug development. Molecular glue drugs represent the key that could unlock this "Pandora's box" of untapped therapeutic potential and immense market value.
As an emerging therapeutic strategy that "glues" proteins together to regulate their function, molecular glues enable targeted protein degradation (TPD), transforming undruggable targets into druggable ones and thereby facilitating drug development. Since the beginning of this year, numerous Chinese and global pharmaceutical companies—including Degron Therapeutics, Kangpu Biopharmaceuticals, GenFleet Therapeutics, Jiayue Pharmaceutical, Wanchun Hongji,Humanwell Likang Pharmaceutical, BMS, C4 Therapeutics, Monte Rosa, Nurix Therapeutics, Pin Therapeutics, and Revolution Medicines—have made remarkable progress in areas such as hematologic malignancies, solid tumors, and inflammation through their clinical pipelines based on molecular glue technology.
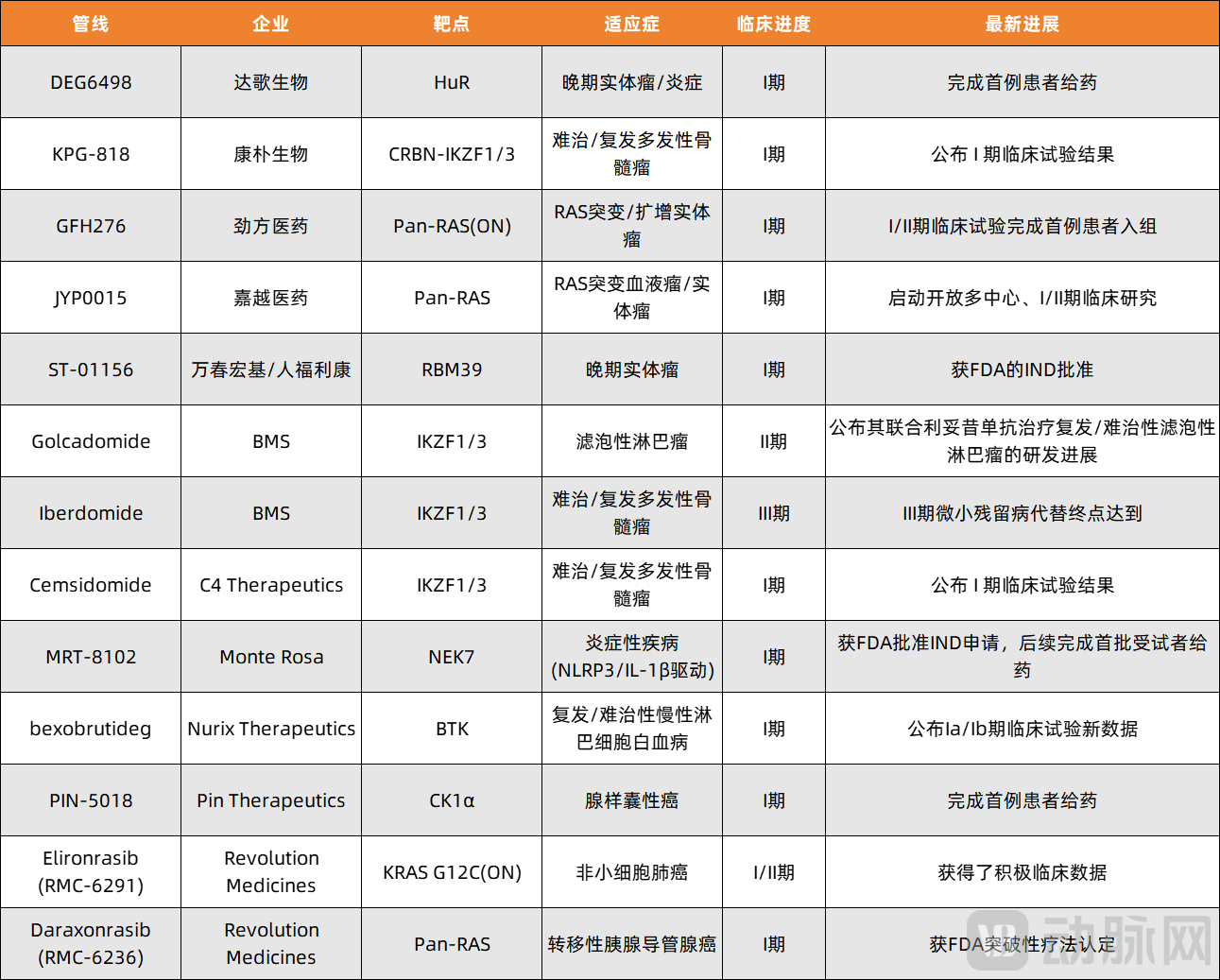
Figure 1. According to incomplete statistics from VCBeat, molecular glue pipelines made progress in clinical fields in 2025.
It is evident that molecular glues are reshaping the boundaries of drug discovery across multiple fronts—from inducing protein degradation to modulating signaling pathways, and from hematologic malignancies to solid tumors—through diverse technological approaches and across various disease areas.
In 2025, Molecular Glue Enters the "Multi-Target, Multi-Indication" Development Stage
The breakthrough in molecular glue research in 2025 goes far beyond a mere increase in pipeline numbers. It marks a strategic transition from "peripheral innovation" to a "mainstream narrative," fundamentally reshaping the industry's approach to target selection and investment value assessment.
In terms of target selection, the scope of molecular glue targets has rapidly expanded from traditional transcription factors (such as IKZF1/3) to kinases (e.g., CK1α), RNA-binding proteins (e.g., HuR), GTPases (e.g., RAS), and translation termination factors (e.g., GSPT1), among others. This diversity is not coincidental; it reveals the universal applicability of molecular glue technology—namely, designing or discovering small molecules to "rewire" intracellular protein interaction networks.
More strategically, breakthrough progress by companies such as Revolution (RMC-6291, target: KRAS), Degron Therapeutics (DEG6498, target: HuR), and Seed (ST-01156, target: RBM39) in novel target areas has transcended individual successes. Collectively, these achievements form a robust "proof-of-concept chain," signaling to the entire industry that the "molecular glue logic," based on the modulation of protein interfaces, is a feasible and potentially optimal pathway to unlock the treasure trove of targets that lack traditional active pockets.
These emerging targets correspond to the expansion into new therapeutic areas. Molecular glue drugs are progressively extending beyond their initial focus on hematologic malignancies to solid tumors, autoimmune diseases, neurological disorders, and more. Advances in target and indication expansion for molecular glues have not only directly stimulated research and development projects targeting previously undruggable proteins but have also indirectly attracted multinational corporations (MNCs) such as AbbVie, Eli Lilly, Roche, Gilead, and Novartis to rapidly enter the field through licensing deals initiatives. Already this year, several major collaborations have been established in this area, with cumulative deal values exceeding USD 11 billion.
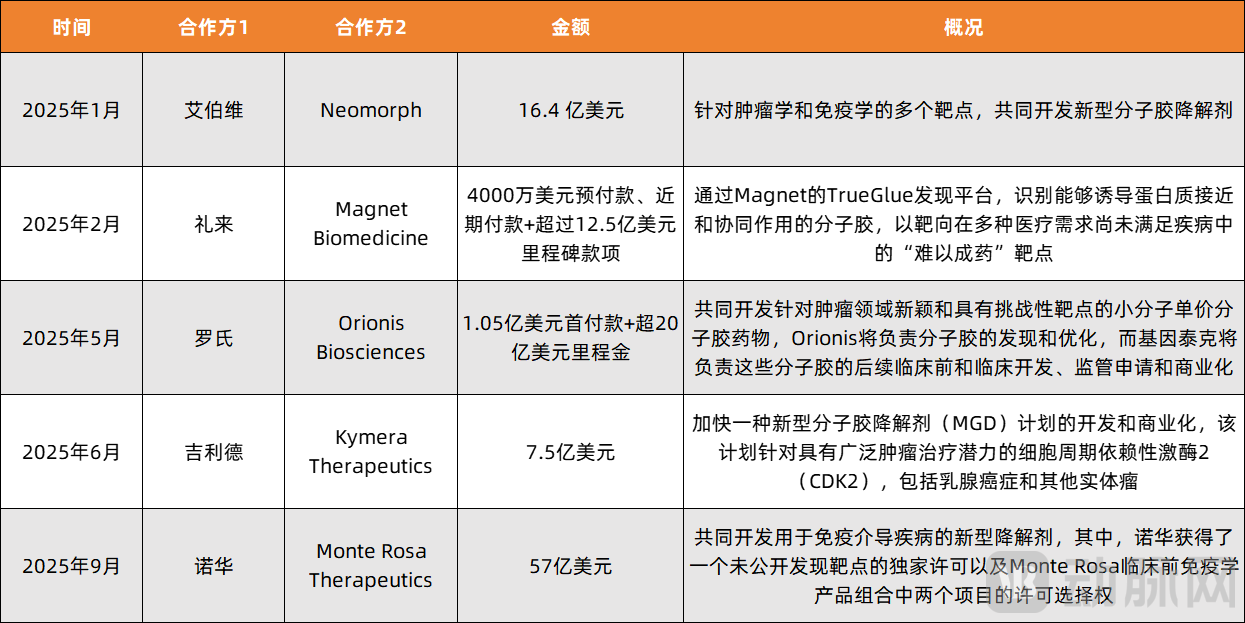
Figure 2. According to incomplete statistics from VCBeat, licensing deals achievements in the molecular glue field by 2025
In summary, in 2025, the field of molecular glues continues the trend of active licensing deals seen in 2024, demonstrating breakthroughs across multiple targets and disease areas. The core driving force lies in its systematic proof that it is not merely a "tactical" tool limited to specific targets (such as CRBN-dependent degradation), but rather a universal drug action paradigm capable of providing novel solutions for diverse protein functions and disease mechanisms.
Therefore, the landscape in 2025 depicts a transformation of the industry paradigm: molecular glues are rapidly evolving from an "emerging technology" that required additional validation into one of the "standard options" that must be considered when evaluating any major disease target. This marks both a milestone in its technological maturity and the starting point of its profound impact on the fundamental logic of global drug discovery and development.
Multi-Dimensional Technology Integration Propels Molecular Glue R&D into an Engineering Development Phase
The fundamental driver behind the rapid advancement of molecular glues is the breakthrough and application of drug screening technologies.
Traditional drug screening operates like searching for a specific target in an unknown space, with its core strategy focused on achieving high-affinity binding to a single target—akin to finding a key that perfectly fits a specific lock. In contrast, the mechanism of molecular glues requires them to simultaneously engage in weak interactions with two proteins and effectively induce their proximity. This process can be likened to finding two keys that open two seemingly unrelated locks, thereby constructing a novel molecular interaction pattern.
This essential difference renders traditional screening methods largely ineffective for molecular glue development, much like using a hammer where tweezers are needed, completely mismatched to the requirements of molecular glue research. This critical challenge has urgently driven the emergence of new screening concepts and technological combinations, forming a significant barrier that must be overcome in the journey of molecular glue development.
Amid this technological transformation, the "breadth-depth" dual-track strategy has emerged as a key solution.
On the breadth front, DNA-encoded libraries (DEL) enable the efficient encoding, storage, and screening of vast chemical collections by covalently linking small-molecule compounds to unique DNA sequences. DEL technology has become a vital tool in drug discovery, particularly in identifying novel hit compounds and exploring challenging targets such as membrane proteins and protein-protein interaction interfaces. It is now widely applied in molecular glue development programs.
For instance, WuXi AppTec's diverse DEL libraries currently encompass over 50 billion structurally distinct small molecules, each tagged with a unique DNA barcode. These molecules function like distinct individuals in chemical space, allowing researchers to rapidly identify and investigate them. In the search for novel molecular glue targets, DEL libraries can impartially scan this expansive chemical space at minimal cost to uncover initial clues for ternary relationships among E3 ligases, ligands, and target proteins, thereby broadening the possibilities for molecular glue discovery.
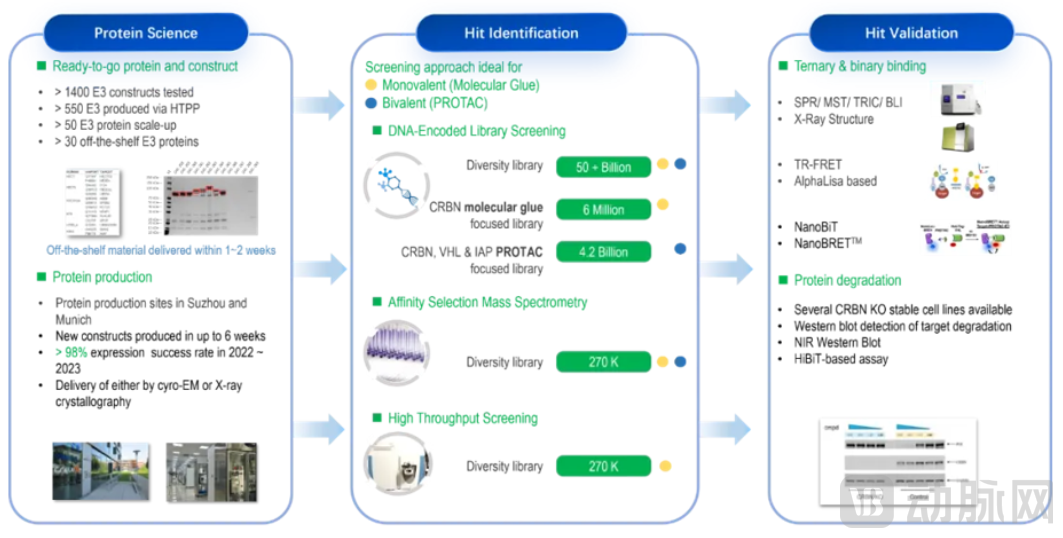
Figure 3. WuXi AppTec Molecular Glue Technology Platform
In the in-depth exploration of known molecular glue systems, focused compound libraries leverage existing structural knowledge for targeted design. A prime example is the immunomodulatory drug (IMiDs) compound library targeting the E3 ligase cereblon (CRBN). Through systematic chemical modifications of the protein interaction regions of CRBN, WuXi AppTec constructed a refined library of approximately 6 million compounds. These compounds are specifically designed and optimized based on existing molecular glue structural insights, significantly increasing the probability of discovering molecular glues with high affinity and specificity, thereby enhancing screening efficiency for targets with well-defined structure-activity relationships.
Beyond the dual-track strategy, multi-technology integration has become indispensable in molecular glue development, establishing a complete technological loop from "hit discovery" to "lead optimization." Affinity selection mass spectrometry (ASMS), as a label-free screening technology, precisely identifies potential molecular glue candidates capable of promoting protein-protein interactions by comparing mass spectrometry signals of molecules in single-protein versus dual-protein environments, effectively screening vast compound libraries for molecules of potential value.
High-throughput screening (HTS) technology operates in an automated "one-compound-per-well" format, combined with functional assays such as protein binding or degradation capabilities. This enables rapid screening of bioactive candidate molecules, greatly improving both screening efficiency and accuracy, ensuring that only the most promising molecules advance to subsequent optimization stages.
Structural biology, by resolving the three-dimensional structures of protein-molecular glue complexes, provides critical structural information for molecular glue optimization. It helps researchers gain in-depth insights into the interaction patterns between molecular glues and proteins, enabling targeted structural refinement and offering essential guidance for enhancing molecular glue performance.
The deep integration of these technologies signifies that molecular glue development has entered an "engineered, multi-dimensional collaborative" phase. In this stage, various technologies work synergistically, complementing each other's strengths to collectively drive the transition of molecular glues from fundamental laboratory research to clinical application, laying a solid technological foundation for the research and development of molecular glue-based therapeutics.
Molecular Glue Achieves 100% Six-Month Survival Rate for Cancer Patients
In the development of numerous molecular glue novel drugs in 2025, companies from China and other countries have achieved breakthrough progress across different targets and disease areas. Their clinical data and mechanisms of action provide robust evidence for the therapeutic potential of molecular glue therapies.
Overseas, leading molecular glue companies such as Pin Therapeutics, Revolution Medicines, Monte Rosa Therapeutics, and C4 Therapeutics have all reported positive advancements this year. Take Revolution Medicines' Elironrasib (RMC-6291) and Daraxonrasib (RMC-6236) as examples.
In June, Revolution Medicines announced that the FDA granted Breakthrough Therapy Designation (BTD) to Daraxonrasib (RMC-6236)—a multi-selective inhibitor targeting RAS(ON)—for the treatment of previously treated pancreatic cancer with KRAS G12 mutations. This BTD is based on promising early clinical evidence observed in patients with pancreatic ductal adenocarcinoma (PDAC): a median progression-free survival (PFS) of 8.8 months (significantly surpassing the 3–5 months seen with conventional chemotherapy), a 6-month survival rate of 100% (compared to approximately 60% in the control group), an objective response rate (ORR) of 36% (with notable tumor shrinkage in some patients), and a favorable safety profile with no severe adverse events reported. The phase 3 clinical study for RMC-6236 is currently underway, with enrollment expected to be completed in 2026.
Elironrasib (RMC-6291) is a RAS(ON) G12C selective inhibitor developed by Revolution Medicines and belongs to the category of non-degradative molecular glues. In October, Revolution Medicines reported encouraging clinical data for Elironrasib in patients with KRAS G12C-mutated non-small cell lung cancer (NSCLC) who had previously received KRAS(OFF) G12C inhibitor therapy: an ORR of 42%, a disease control rate (DCR) of 79%, a median duration of response of 11.2 months, a median PFS of 6.2 months, and a 12-month overall survival rate of 62%. Currently, Elironrasib is being explored in combination with immunotherapies (such as pembrolizumab) and other targeted agents (e.g., SHP2 inhibitors) to further enhance efficacy. The company also plans to advance it into phase 2 and phase 3 clinical trials to validate its value in first-line treatment and across additional tumor types.
In China, several innovative pharmaceutical companies have also made breakthrough progress in the molecular glue field recently.
For instance, in December, Degron Therapeutics announced that DEG6498—the world's first molecular glue degrader targeting the first-in-class target HuR (Human antigen R)—had dosed its first clinical trial participant in China on November 25. HuR is an RNA-binding protein that plays a critical role in cancer, inflammation, metabolic diseases, and other pathological processes, and was previously considered an undruggable target. Leveraging its proprietary molecular glue discovery platform and innovative technologies, Degron Therapeutics successfully addressed this challenge. DEG6498 is a potent, orally bioavailable small-molecule molecular glue degrader that induces interaction between the E3 ubiquitin ligase Cereblon (CRBN) and HuR, thereby promoting targeted degradation of HuR. As the first HuR-targeting molecular glue degrader globally, DEG6498 holds promise to address urgent unmet medical needs across multiple therapeutic areas.
In November, Kangpu Biopharmaceuticals released phase I clinical trial results for its molecular glue degrader epaldeudomide (KPG-818) in hematologic malignancies. KPG-818 is a CRL4-CRBN E3 ubiquitin ligase complex modulator with high affinity for CRBN. In patients with relapsed or refractory multiple myeloma who had previously received at least two immunomodulatory imide drugs, one proteasome inhibitor, and one anti-CD38 monoclonal antibody, the combination of KPG-818 and dexamethasone achieved an ORR of 50% and a DCR of 94%. It exerts broad-spectrum immunomodulatory, anti-angiogenic, and anti-tumor effects by efficiently degrading the zinc-finger transcription factors Aiolos (IKZF3) and Ikaros (IKZF1).
Additionally, other Chinese pharmaceutical companies such as GenFleet Therapeutics, Jiayue Pharmaceutical, Wanchun Hongji, and Humanwell Likang Pharmaceutical have also made significant progress in the molecular glue field this year.
The simultaneous focus on molecular glue pipelines by companies in China and other countries is driven by the high technical barriers in the field, relatively limited competition, and the expanding application of molecular glues from oncology to broader disease areas. Moreover, to date, only three molecular glue drugs—thalidomide, lenalidomide, and pomalidomide—have reached commercialization. The combination of high technical barriers and a largely untapped market provides Chinese startups with a platform to compete alongside international leaders and offers a strategic window for innovative Chinese companies to accelerate their growth. As clinical trial data continue to emerge, several promising domestic molecular glue pipelines in China are expected to stand out, potentially securing greater influence for Chinese pharmaceutical companies in the global market.
Future Outlook: What Are the Next Decade's Challenges for Molecular Glues?
Despite the remarkable progress achieved in the field of molecular glues in 2025, significant challenges remain on the path forward.
Target selectivity stands as one of the foremost challenges for molecular glues. Within complex biological systems, ensuring that molecular glues act precisely on the intended target without engaging in unintended interactions with non-target proteins is crucial for safe and effective therapy. However, current molecular glue technologies still face limitations in target selectivity, which may lead to off-target effects and subsequent adverse reactions. For instance, while degrading the target protein, certain molecular glues might inadvertently affect the stability of other proteins involved in normal physiological functions, posing potential harm to the organism.
Off-target toxicity is another issue that cannot be overlooked. Even if a molecular glue can relatively accurately target its intended protein, its mechanism—involving the induction of protein-protein interactions—may trigger a cascade of unpredictable cellular reactions, leading to toxicity. Such off-target toxicity can not only limit the dosage and efficacy of molecular glue drugs but also hinder their further clinical application. Balancing enhanced activity with minimized off-target toxicity remains a critical challenge for researchers.
A deep understanding of resistance mechanisms is equally vital. As the clinical use of molecular glue drugs increases, disease-related cells such as tumor cells may gradually develop resistance, reducing therapeutic efficacy. Investigating the mechanisms underlying resistance to molecular glues is essential for developing effective strategies to prolong their clinical utility. Currently, research on resistance mechanisms in molecular glues remains relatively limited and requires further exploration.
Developing molecular glues targeting E3 ligase systems beyond CRBN represents another key focus for future research. Most current molecular glue development centers on CRBN-based systems, which, to some extent, restricts their scope of application and potential. Developing molecular glues that engage other E3 ligases could not only expand target options but also offer new therapeutic approaches for refractory diseases. However, due to significant structural and functional differences among E3 ligases, developing molecular glues for non-CRBN systems poses considerable technical challenges.
In future clinical exploration, combination strategies that integrate molecular glues with immunotherapies and targeted therapies hold great promise for synergistic effects. Immunotherapy activates the body's own immune system to combat diseases such as cancer, targeted therapy acts precisely on specific targets in tumor cells, while molecular glues can modulate cellular signaling pathways by degrading key proteins. Combining these three approaches could attack diseases from multiple angles, potentially improving treatment outcomes. For example, in cancer therapy, molecular glues could be combined with immune checkpoint inhibitors. On one hand, molecular glues could degrade immunosuppressive proteins in tumor cells to enhance immune responses; on the other hand, checkpoint inhibitors could activate the immune system to better recognize and attack tumor cells, achieving synergistic therapeutic effects.
With the ongoing advancement and integration of tools such as artificial intelligence (AI) and structural biology, the rational design and optimization of molecular glues are expected to become more efficient. AI can analyze and learn from vast datasets to predict interactions between molecular glues and target proteins, providing guidance for design. Structural biology, by resolving three-dimensional structures of molecular glue-protein complexes, can offer deep insights into their mechanisms, enabling targeted optimization. The application of these tools will help transition molecular glue discovery from "serendipity" to "systematic development," accelerating the drug development process and providing patients with more treatment options.
Conclusion
In 2025, molecular glues are no longer merely scientific concepts confined to the laboratory; they are gradually emerging as new options in clinical therapy. From rare tumors to common mutations, and from degradation to modulation, molecular glues are expanding the dimensions of drug action. This progress is driven by integrated R&D platforms, multidimensional screening technologies, and cross-disciplinary collaboration. In the future, as more molecular glues advance to late-stage clinical trials and even reach the market, we may witness the emergence of a cohort of "glue drugs" that truly transform the therapeutic landscape. This also marks a critical step in drug innovation, shifting from "following" to "leading."
