Infinity Neuro completes Series A financing to accelerate innovation and commercialization of neurovascular surgical medical devices
Recently, Infinity Neuro - a medical device company dedicated to providing innovative solutions for neurovascular surgery - announced the completion of a Series A financing round worth hundreds of millions. This round was led by GTJA Investment Group, with joint investments from multiple institutions including Guoyuan Equity and Jolmo, among others. The collective funding is aimed at accelerating the company's innovative product development and commercialization process, providing neurointerventional specialists with superior treatment options.

Infinity Neuro primarily focuses on the research, development, production, and commercialization of medical devices in the cerebrovascular field. The company is committed to collaborating closely with neurologists to address unmet clinical needs in neurointervention, with product offerings spanning two core therapeutic areas: hemorrhagic and ischemic stroke. The core team consists of professionals with backgrounds in multinational medical device companies and a track record of multiple successful entrepreneurial ventures. They possess extensive expertise in the development, clinical validation, industrialization, and iterative enhancement of neurointerventional medical devices, with a dedicated focus on both hemorrhagic and ischemic stroke markets.
Infinity Neuro is supported by an expert committee comprising globally leading neuroscientific authorities, providing robust academic backing for the company's worldwide technological innovation. This enables the realization of its core value proposition: "from the doctors, by the engineers, for the patients," ultimately benefiting more stroke patients around the world. Infinity Neuro's flagship product, the HUB Navigation-Assisted Reperfusion System, is a global innovation. It has already completed dozens of First-in-Man (FIM) procedures in Europe and the United States, significantly reducing traditional interventional thrombectomy time to approximately 10 minutes—a breakthrough advancement in the field of ischemic stroke intervention in recent years.
With a global market perspective, Infinity Neuro has successfully obtained CE marking and FDA approvals for multiple medical devices across cardiovascular, cerebrovascular, and peripheral vascular fields. The company has established sales channels in China, the United States, Western Europe, India, Brazil, and other regions. This global outlook enables Infinity Neuro to develop differentiated and competitive product pipelines tailored to the specific needs of diverse markets.
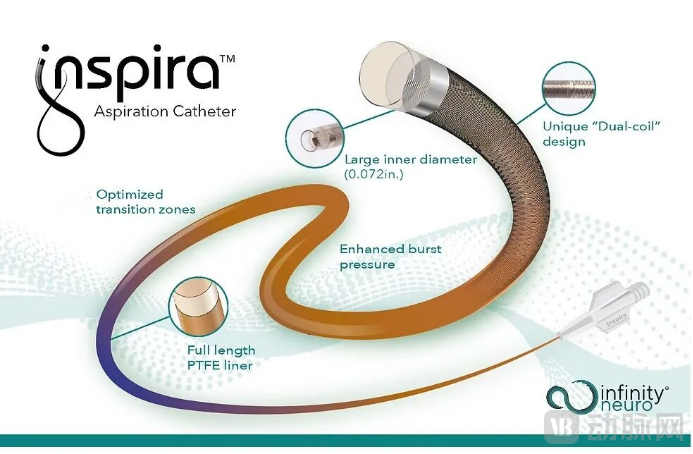
Infinity Neuro's first product, the Inspira Aspiration Catheter, obtained a CE mark as a Class III medical device under the new EU Medical Device Regulation (MDR) in January 2023 and was subsequently launched in the European market for the treatment of acute ischemic stroke. It is the first neurovascular device approved in Europe under this updated regulatory framework. The Inspira Aspiration Catheter offers enhanced operability, providing superior navigation and aspiration performance. It is indicated for the treatment of acute ischemic stroke caused by large vessel occlusion (LVO), enabling rapid access to target vessels, effective thrombus aspiration, restoration of cerebral blood flow, and reduction of brain injury. In 2024, the Inspira Catheter received market approval from the National Medical Products Administration (NMPA) of China.
