YESight Medical raises tens of millions in Series A+, advancing leadership in high-end ophthalmic diagnostics
Recently, Yaoshi (Suzhou) Medical Technology Co., Ltd. ("YESight Medical") has completed a Series A+ financing round totaling tens of millions of RMB. This round was strategically led by Hua Capital (元禾璞华), with follow-on investment from Haihong Jinsu (海鸿金栗). InvesTarget acted as the exclusive financial advisor. The raised funds will be primarily used to accelerate the global commercialization of YESight Medical's core product—the ultra-widefield scanning laser ophthalmoscope (SLO)—and to advance the development of multiple innovative ophthalmic optical devices, solidifying its leading position in the industry.
YESight Medical is an innovative ophthalmic medical device company that integrates R&D, production, sales, and AI big data research into a unified innovative ophthalmic optics platform. The company boasts a seasoned technical team with nearly 20 years of collective experience from global Fortune 500 companies and renowned international ophthalmic device firms, possessing deep expertise in core image processing, optical design, industrialization, and engineering. Currently, YESight Medical has established production and R&D centers in Suzhou, Nantong, and Japan. Based on its high-end ophthalmic optical platform, the company is dedicated to pioneering innovative ophthalmic diagnosis and screening technologies.
1Leads with Technological Innovation and Multi-dimensional Advantages
The global and Chinese ophthalmic disease landscape is currently characterized by two prominent features and developmental trends that are profoundly shaping the direction of eye health:
First, the patient population continues to expand with a noticeable trend toward disease generalization. As population aging deepens globally and in China, the prevalence of age-related fundus diseases such as age-related macular degeneration (AMD), diabetic retinopathy (DR), and pathological myopia is persistently rising. Simultaneously, evolving modern lifestyles are driving a continuous increase in the population affected by chronic diseases like diabetes and hypertension, which are prone to causing severe fundus complications.
Second, diagnostic and treatment needs are undergoing a structural shift toward early intervention and broader market penetration. Historically, fundus examination resources were predominantly concentrated in major hospitals within large cities, leaving screening demands in primary care and underserved markets chronically unmet. Now, however, rising public health awareness and the widespread adoption of the "early screening, early diagnosis, early treatment" principle are driving rapidly surging demand for efficient and convenient fundus screening in primary care institutions and physical examination centers.
Based on these trends, the fundus examination sector is embracing unprecedented growth potential. The core drivers include:
First, there remains substantial unmet clinical demand in fundus screening. While China's potential fundus disease patient population numbers in the hundreds of millions, the current coverage rate of standardized screening and follow-up remains low, creating a significant diagnostic gap.
Second, traditional fundus camera technology suffers from multiple limitations, creating urgent market need for innovative solutions. Current mainstream traditional fundus cameras still face numerous clinical challenges: limited field of view coverage, strong dependency on pupil dilation, high operational thresholds coupled with insufficient imaging stability, and lack of multimodal imaging evaluation capabilities. These shortcomings make them poorly suited for contemporary screening needs emphasizing "early detection, convenience, and precision." This technological gap creates substantial market space for YESight Medical's new-generation devices featuring ultra-widefield imaging, non-mydriatic operation, and rapid imaging capabilities to provide replacement and upgrade solutions.
Third, dual drivers of policy and technology. National emphasis on chronic disease management and tiered healthcare systems, combined with the maturation of artificial intelligence (AI) technology in auxiliary diagnostics, are jointly propelling fundus examination equipment to become a critical gateway for eye health and even systemic health management.
According to forecasts from Frost & Sullivan, the Chinese ophthalmic medical diagnostic device market is projected to reach RMB 4.6 billion and RMB 8.0 billion in 2025 and 2030, respectively. The compound annual growth rates (CAGR) from 2020 to 2025 and from 2025 to 2030 are estimated at 18.2% and 11.8%. As the core link for early screening and diagnosis of ophthalmic diseases, fundus examination represents a golden sector experiencing rapid growth, embodying both social value and commercial potential. Currently, YESight Medical has established a multi-dimensional leading advantage in this field.
2Breaking the Import Monopoly of High-End Ophthalmic Equipment
To address the shortcomings of traditional fundus cameras, YESight Medical has developed the Yetsea series of ultra-widefield scanning laser ophthalmoscopes (models 100, 200, and 300). Building upon the foundation of "ultra-widefield imaging and non-mydriatic operation," the series is progressively advancing toward "intelligentization, multimodality, and high performance," aiming to provide the most suitable solutions for medical institutions across different tiers.
Specifically, the Yetsea series addresses the pain points of traditional technology across six key dimensions:
First, extensive imaging range. YESight Medical's ultra-widefield SLO operates on the fundamental principle of laser scanning technology, capturing a 168° fundus range in a single image—a significant improvement over the 30°-50° range of traditional fundus cameras. The company's proprietary infrared panoramic imaging technology (patent pending) achieves a single-shot range exceeding 270°, thereby fully covering the entire retinal area and substantially reducing the missed diagnosis rate for peripheral retinal lesions.
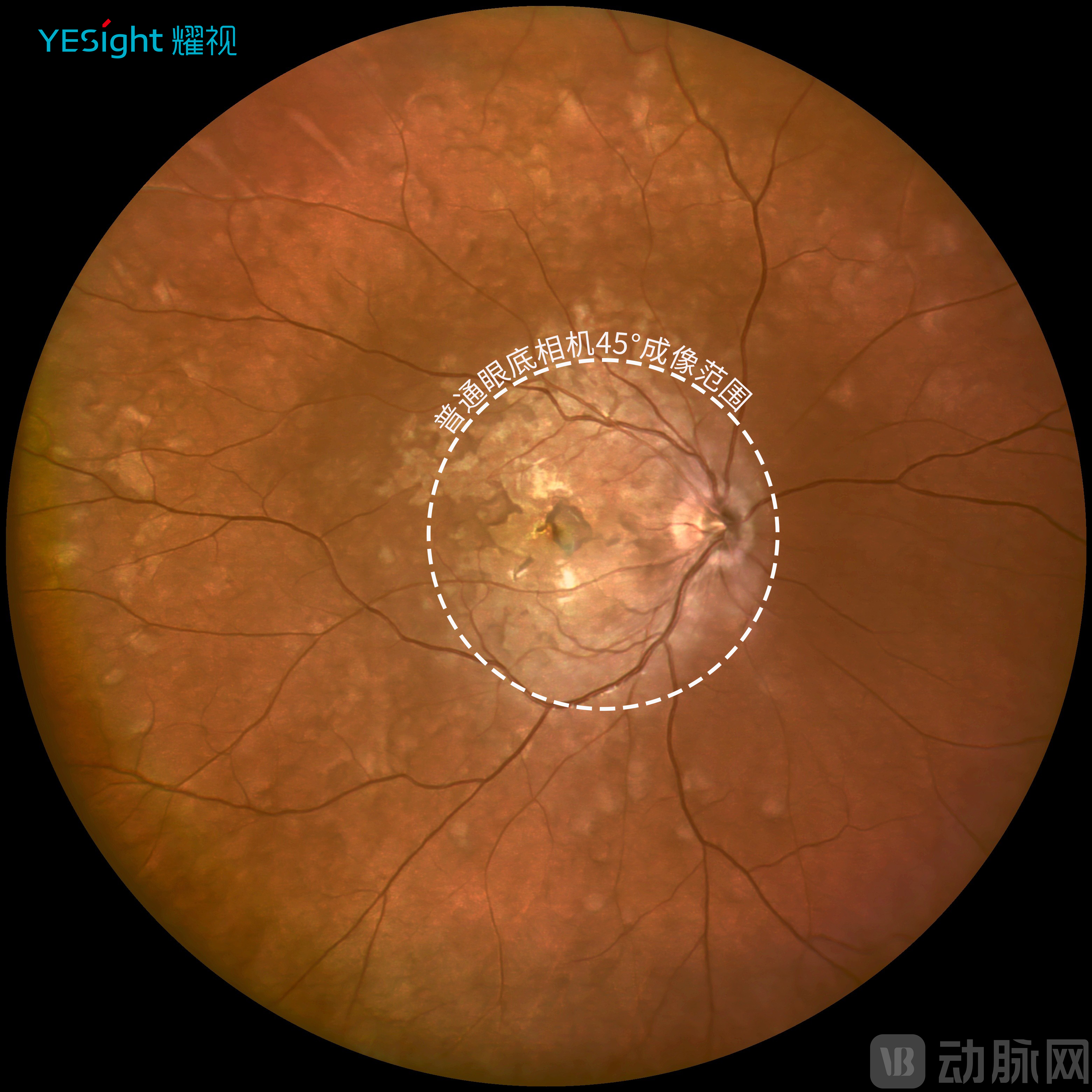
Traditional 45° Color Fundus Photo vs. YESight Medical's 168° Ultra-Widefield Image
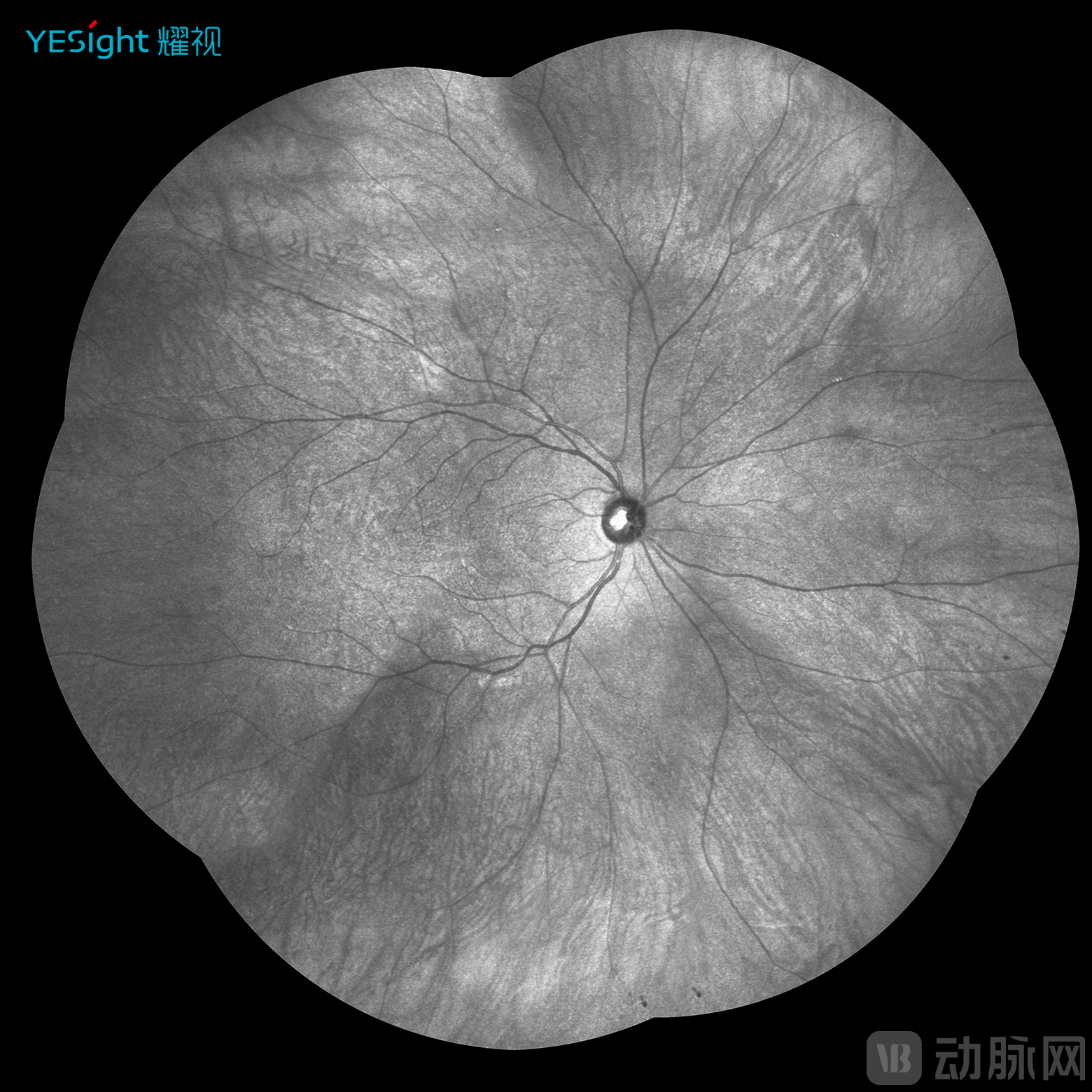
YESight Medical Proprietary Infrared Panoramic Fundus Image (Static)
Second, no pupil dilation required. The device requires a minimum pupil size of only 1.7mm, eliminating patient discomfort post-dilation while saving time and improving diagnostic efficiency. This is particularly significant for patients who cannot undergo dilation (e.g., glaucoma) or for whom dilation is difficult (e.g., diabetic patients).
Third, fast imaging speed. The ultra-widefield SLO captures images in an extremely short time, significantly reducing examination duration and enhancing patient comfort. Notably, YESight Medical's industry-first integrated auto-alignment technology substantially reduces actual imaging time.
Fourth, high image resolution. YESight Medical's ultra-widefield SLO achieves a maximum resolution of 4.9μm with 3120×3120 pixels, capturing fundus details beyond the capability of conventional fundus cameras. This provides clinicians with clearer, more detailed evidence for diagnosis.
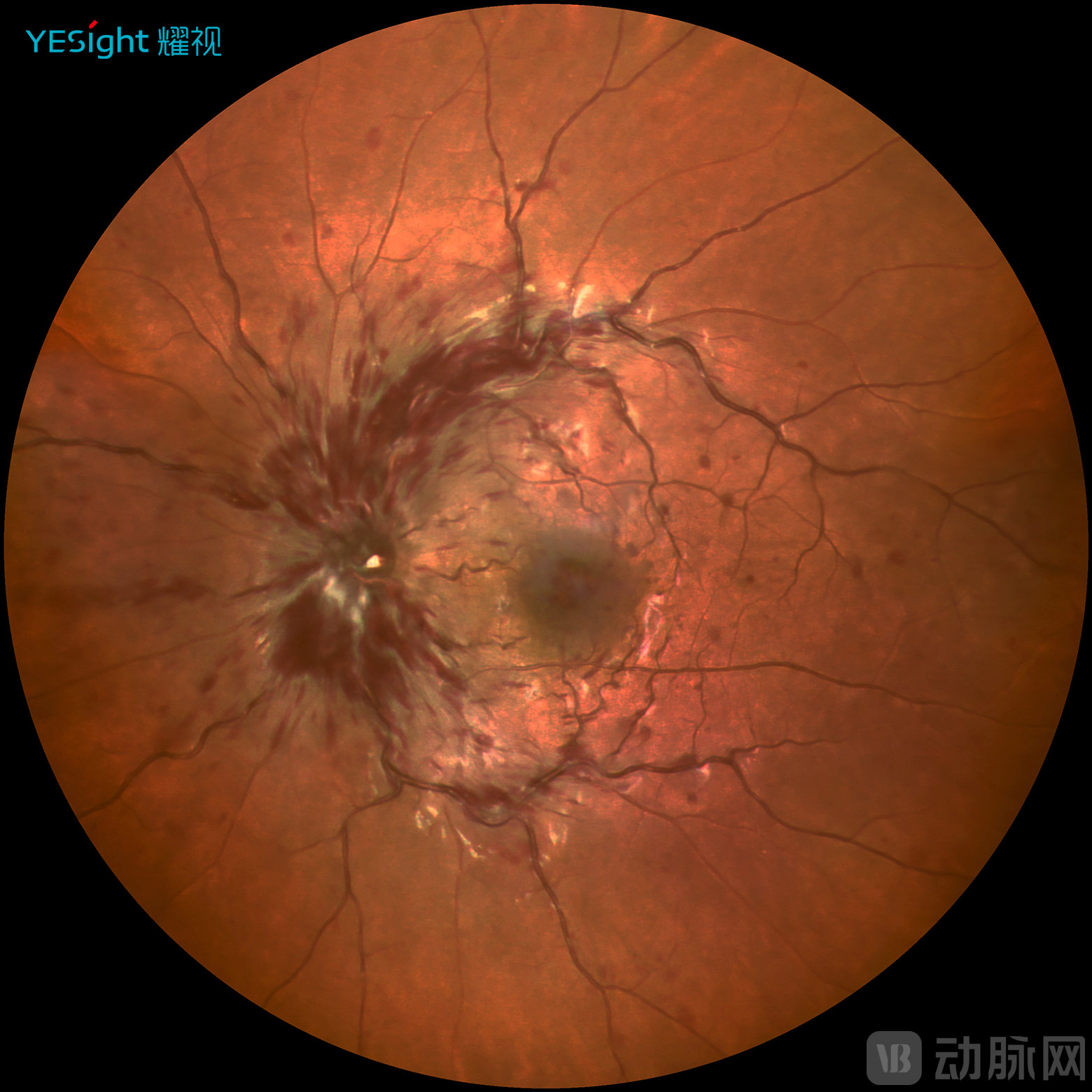
Color Photography
Fifth, superior penetration capability. For patients with cloudy refractive media (such as cataracts), traditional fundus cameras struggle to obtain clear fundus views through the opacified lens. YESight Medical's ultra-widefield SLO utilizes four laser wavelengths (red, blue, green, and infrared) for imaging, providing enhanced penetration that can visualize through 85% of cataracts—even obtaining clear fundus images through Grade 4 nuclear cataracts. This significantly expands the applicable scope of fundus examinations.
Sixth, multimodal imaging implementation. YESight Medical's SLO introduces multimodal imaging capability, moving beyond conventional color fundus photography. By fully leveraging the core characteristics of laser illumination, the system captures autofluorescence images and fluorescein angiography with higher lesion contrast compared to traditional cameras, providing superior diagnostic information.
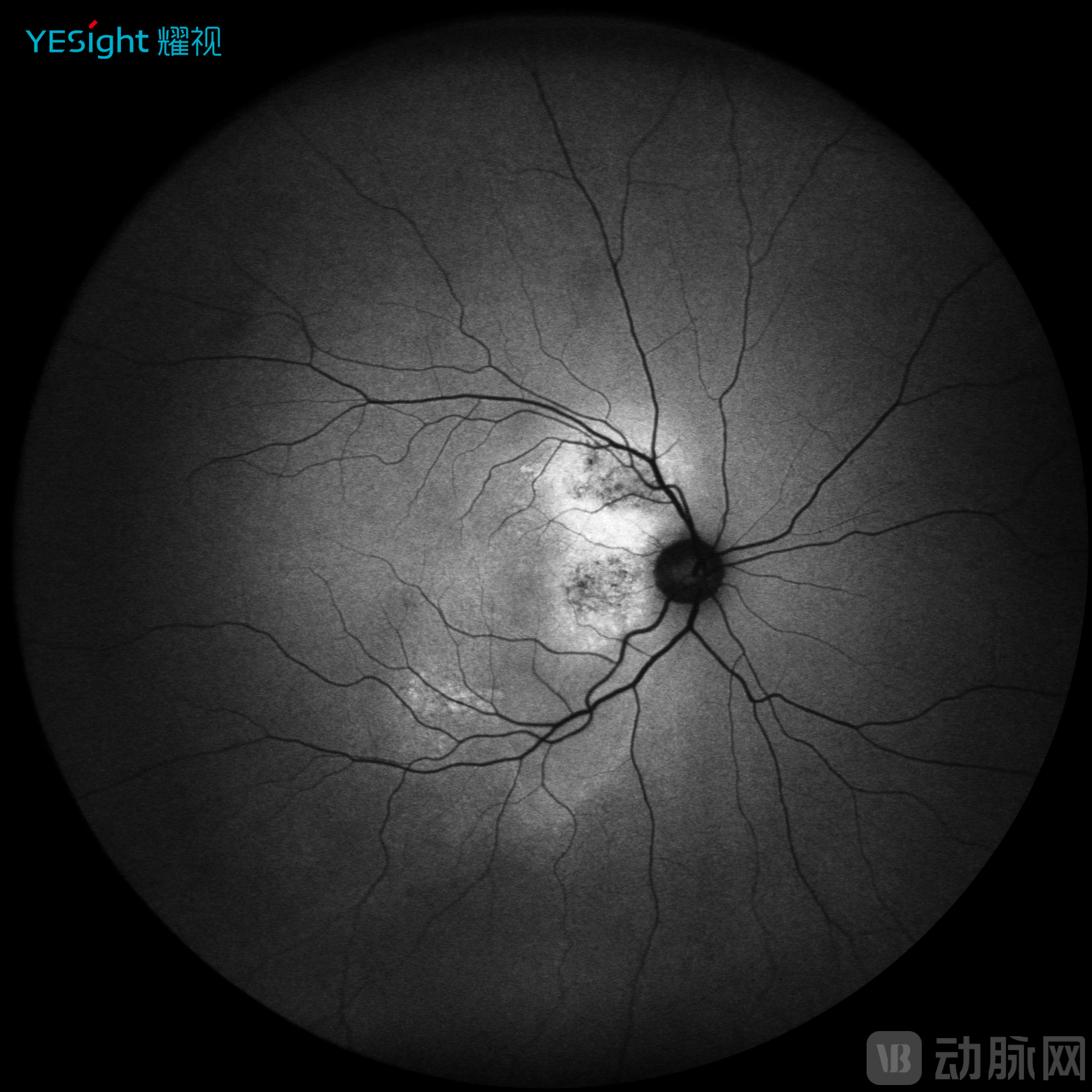
Autofluorescence
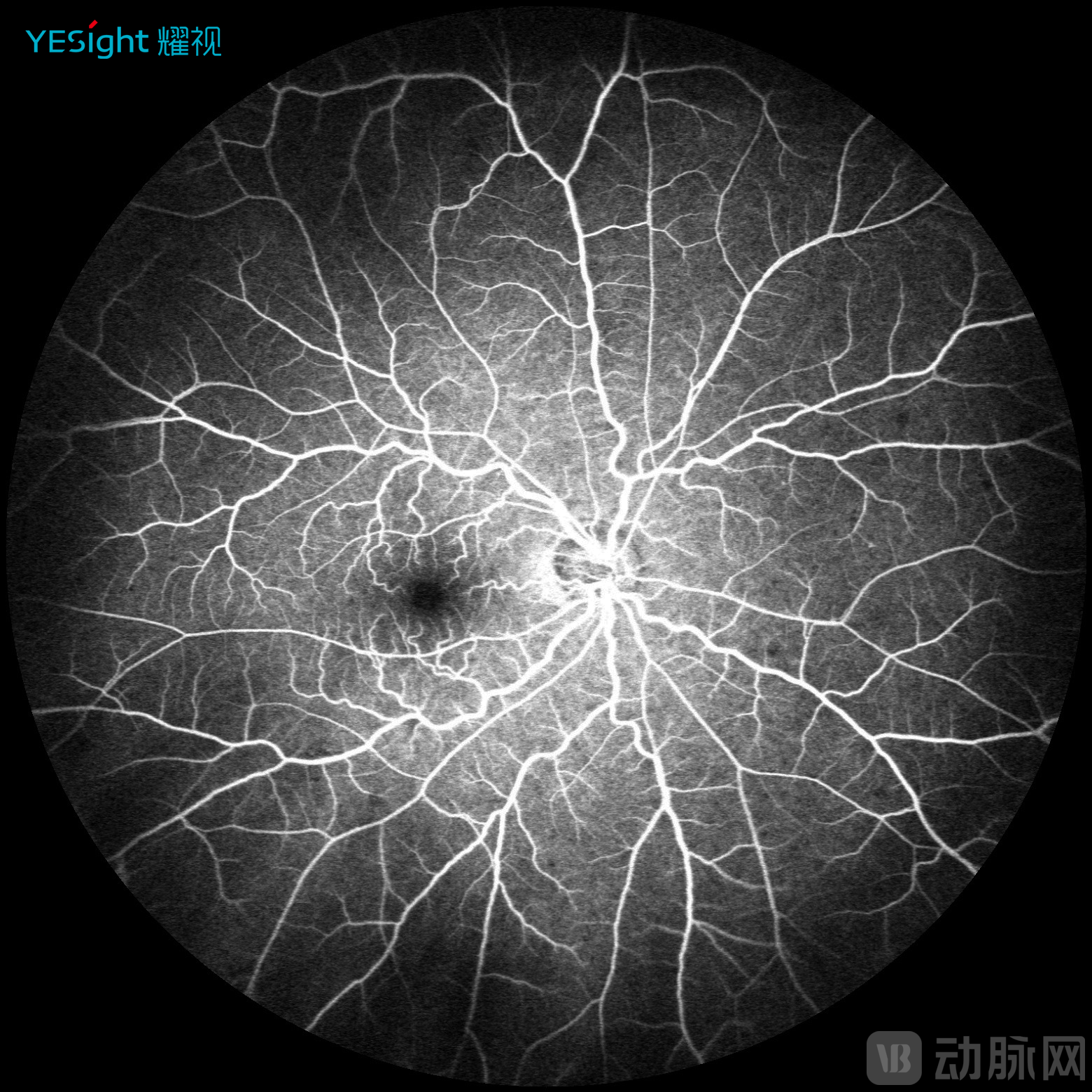
FFA Angiography
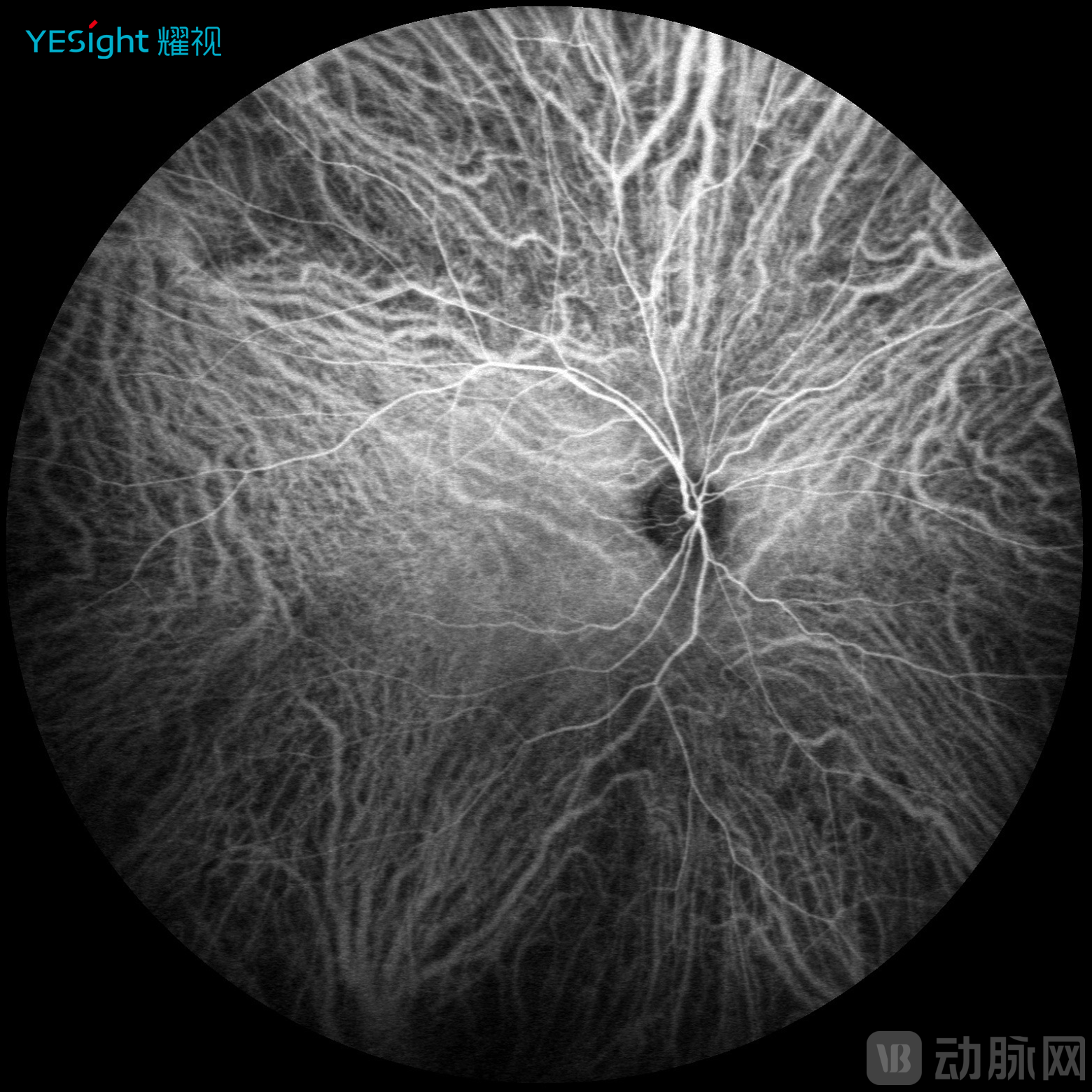
ICGA Angiography
Furthermore, YESight Medical's proprietary "Anywhere Door" function (patent pending) enables retrospective review of dynamic imaging sequences corresponding to any point on the color fundus photo. This represents a revolutionary upgrade from static 2D images to dynamic visualization, establishing an entirely new diagnostic paradigm in fundus examination.
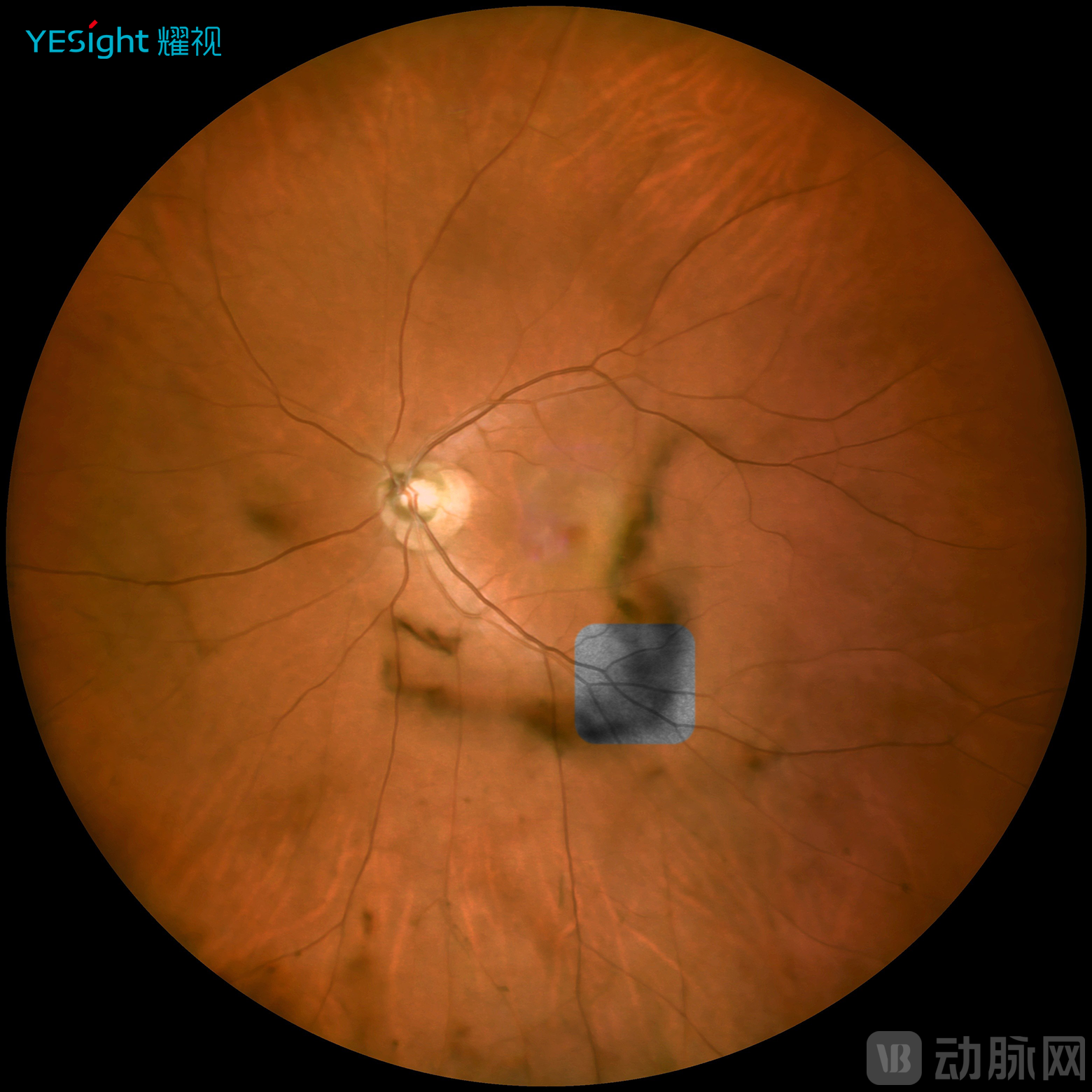
YESIGHT's Unique "Any Door" Function
The launch of the Yetsea series ultra-widefield scanning laser ophthalmoscopes marks a critical milestone in YESight Medical's mission to achieve "high-end ophthalmic equipment made in China."
First, through independent innovation, its core performance has reached internationally advanced levels, achieving parity with leading imported brands in key specifications. Second, it provides hospitals with a new "high-quality yet cost-effective" alternative, promoting technological accessibility. Third, YESight Medical has progressively established and refined a localized core supply chain system. This reduces dependency on specific international supply chains, enhances industrial security, and creates advantages in responsiveness and service depth that imported manufacturers cannot match.
Concurrently, during product iteration, YESight Medical has established long-term, in-depth collaborations with top-tier ophthalmic centers and clinical experts across China to deepen clinical co-development. In parallel, the company has implemented a "demand funnel" mechanism, supported by a dedicated clinical applications department that systematically collects, consolidates, and analyzes feedback from hospitals of different tiers. This feedback is directly translated into product feature optimizations, ensuring accurate insight into real clinical needs and effectively addressing pain points.
3Expand Overseas Market Layout
Currently, YESight Medical has initiated its global market expansion, establishing exclusive strategic partnerships with several listed technology companies from Japan and other countries to jointly develop domestic and international ophthalmic markets. This not only signifies the company's successful technology export but also demonstrates that its product value is recognized by major overseas enterprises, laying a solid foundation for rapidly capturing market share worldwide.
YESight Medical anticipates that over the next five years, competition in the high-end ophthalmic equipment sector will evolve into a comprehensive contest involving technological depth, market breadth, and ecosystem development capabilities. In response, the company will continue focusing on genuine clinical needs to develop high-quality ophthalmic screening and diagnostic devices that deliver clear imaging, user-friendly operation, and reliable performance. By integrating AI algorithms, YESight Medical will further deepen its technological moat. Simultaneously, the company will expand its coverage across various ophthalmic diagnostic domains—including the fundus, anterior segment, and refractive conditions—to build a more comprehensive product portfolio. It will actively pursue strategic collaborations with major ophthalmic service providers and global ophthalmic giants, exploring partnerships in market development and technology to accelerate its commercial progression.
