Kailera Therapeutics secures $600M Series B, advancing Hengrui-licensed obesity drug to global trials
On October 14, 2025, Kailera Therapeutics announced the completion of a $600 million Series B financing round. The round was led by Bain Capital Private Equity, with participation from multiple new and existing investors. The proceeds will primarily be used to advance its lead candidate drug, KAI-9531 (internal code HRS9531 at Hengrui Pharmaceuticals), into global Phase III clinical trials. Additionally, the funds will accelerate the development of its portfolio of oral and injectable formulations, strengthening its global expansion in the treatment of obesity and metabolic diseases.
Notably, Kailera's R&D pipeline originated from China's Hengrui Pharmaceuticals, following a development path that evolved from "initial clinical development in China—asset spin-off—independent financing and global development."
Kailera: Originating from Hengrui's authorization, targeting markets outside Greater China
Kailera Therapeutics was established in 2024, with its core assets originating from investigational drugs licensed from Hengrui Pharmaceuticals—a fact explicitly stated in public disclosures. Its lead asset, KAI-9531, is an injectable GLP-1/GIP dual receptor agonist. Currently, Hengrui leads its clinical development within China, while Kailera holds global rights to the drug outside Greater China. This division of responsibilities has endowed the company with an international focus from its inception, distinguishing it from typical early-stage R&D biotechs.
Beyond its core asset, Kailera's pipeline includes the oral small-molecule GLP-1 agonist KAI-7535, an oral formulation of KAI-9531, and a GLP-1/GIP/glucagon triple agonist KAI-4729. The portfolio spans both injectable and oral modalities while expanding into multi-target mechanisms, aiming to balance therapeutic depth with administration convenience. The company explicitly stated in its Series B financing announcement that the proceeds will primarily support advancing KAI-9531 into global Phase III trials and accelerating KAI-7535's transition from Phase II studies in China to international trials.
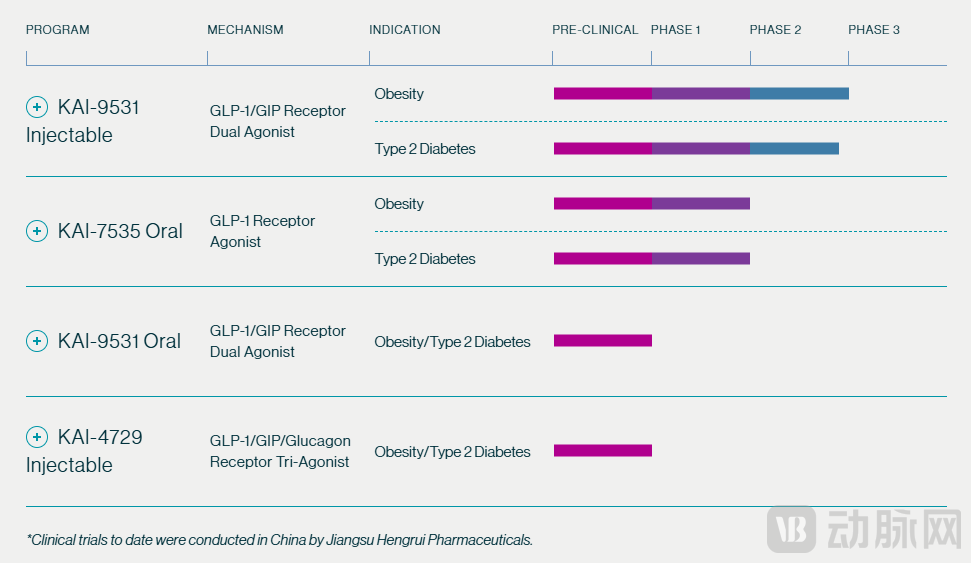
Overview of Kailera's Pipeline
Kailera has assembled a leadership team with deep biopharmaceutical expertise spanning the full spectrum of drug development, commercialization, and corporate management. President and CEO Ron Renaud previously steered the growth and successful acquisitions of companies including Cerevel and Translate Bio. Chief Commercial Officer Jamie Coleman led the commercial launches of blockbuster therapies such as Zepbound and Trulicity. Chief Medical Officer Dr. Scott Wasserman contributed to global regulatory approvals for Repatha and Evenity, while Chief Technology Officer Dr. Doug Bakan brings over 35 years of pharmaceutical research and development experience to the organization.
What has further drawn attention to Kailera is the results from HRS9531's clinical trials in China. In a Phase III study involving 567 participants (531 completed the study), the 6 mg dose group achieved an average weight reduction of 19.2% after 48 weeks. Specifically, 88% of participants lost at least 5% of their body weight, while 44.4% achieved weight loss exceeding 20%. In terms of safety profile, adverse events were primarily mild to moderate gastrointestinal reactions, consistent with previously observed GLP-1 class drugs. Earlier Phase II data demonstrated that the 8 mg dose group could achieve up to 22.8% weight reduction over 36 weeks, without showing signs of reaching a plateau. This characteristic of "sustained weight loss without early plateau" presents a distinct competitive advantage in the current obesity drug landscape, making it a particularly valued feature by both investors and developers.
Kailera's financing trajectory demonstrates notable strategic momentum. In October 2024, the company completed a $400 million Series A round led by Atlas Venture, Bain Capital Life Sciences, and RTW Investments. Through this transaction, Kailera secured ex-Greater China rights to multiple metabolic and obesity programs from Hengrui Pharmaceuticals, while Hengrui obtained approximately 19.9% equity stake in Kailera. Merely one year later, the company closed a $600 million Series B financing led by Bain Capital Private Equity, with proceeds dedicated to advancing its core pipeline through global clinical development and regulatory registration.
Two Large Rounds of Financing in a Year, Kailera Leads the GLP-1 Track
Kailera's ability to progress from a $400 million Series A to a $600 million Series B within a single year reflects the synergistic convergence of capital, technology, and industry drivers.
From a capital perspective, GLP-1 and related mechanisms have emerged as one of the most dynamic therapeutic areas in the global pharmaceutical industry. According to projections from multiple investment banks, the global obesity treatment market is entering a phase of rapid expansion. BMO Capital Markets anticipates the global obesity drug market will reach $150 billion by 2033, while Leerink Partners forecasts the weight-loss drug market will grow to $158 billion by 2032.
Regarding specific drug performance, Eli Lilly's GLP-1/GIP dual receptor agonist tirzepatide (brand name Zepbound) achieved over $4 billion in sales in 2024 alone, with projections exceeding $20 billion by 2030. Such success stories have significantly raised market valuation expectations for differentiated GLP-1 pathways, prompting investors to strategically position themselves in potential competitors at earlier stages. Kailera's ability to secure consecutive investments from Bain Capital and RTW Investments stems from its "Hengrui-validated + global pathway" combination, which is viewed as a structured asset with replicable potential.
The company's technical pathway represents another key factor driving investor interest. Leveraging China's advantages in clinical resources, patient population scale, and trial efficiency, Kailera first completed Phase III studies for HRS9531 domestically before using the data to support global registration strategies. This "China-as-validation-ground" approach enables rapid iteration in efficacy, safety, and dosage exploration, subsequently providing substantiated evidence for communications with the U.S. Food and Drug Administration (FDA). The company has disclosed completion of its End-of-Phase 2 meeting with the FDA and plans to initiate its global Phase III program by year-end.
At the industrial level, this case reflects structural innovation in the globalization pathways of Chinese innovative pharmaceutical companies. Kailera's establishment did not follow the traditional "out-licensing" model, but instead emerged through Hengrui's incubation of an independent NewCo via an "equity + exclusive ex-China licensing" structure. This approach preserves Hengrui's economic interests in the Chinese market while empowering Kailera with autonomous financing capabilities and global regulatory agency engagement capacity - effectively creating a middle path between "in-house development" and "out-licensing."
However, any distributed governance structure carries inherent risks: asset ownership, data sharing, profit distribution, and even future IPO or exit arrangements require meticulous contractual definition. Without such precision, operational friction may emerge during later stages. Furthermore, regulatory requirements concerning data representativeness and safety cannot be overlooked. Although HRS9531 demonstrated 19.2% average weight reduction in its Chinese Phase III trial (6 mg cohort), whether consistent efficacy can be maintained in Western markets remains subject to validation through global trials with multi-ethnic populations.
In summary, backed by substantial funding and innovative development strategies, Kailera has strategically positioned itself in the global metabolic disease treatment landscape by pioneering a globalization model that leverages Chinese clinical data as foundation for international expansion. Over the next two to three years, the company's critical challenges will center on the timely initiation of global Phase III trials, the advancement of Hengrui's NDA (New Drug Application) in China, and the validation progress of its triple agonist and oral small molecule programs.
