AbbVie's TrenibotE files for market approval in China
On October 13, the website of China's Center for Drug Evaluation (CDE) showed that the marketing application for AbbVie's (ABBV.US) TrenibotE has been accepted. The application is classified as a Category 1 new drug. Based on publicly available information and clinical trial progress, the proposed indication is speculated to be for moderate to severe glabellar lines.
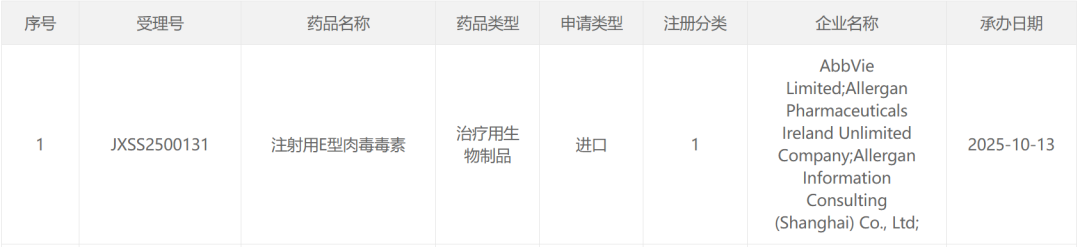
Image from CDE official website
TrenibotE is a first-in-class botulinum neurotoxin serotype E. In April 2025, AbbVie submitted its first marketing application for the product to the U.S. FDA. The submission is supported by clinical data from more than 2,100 patients treated with TrenibotE, including two pivotal Phase III clinical trials (M21-500 and M21-508) evaluating its efficacy in treating moderate to severe glabellar lines, and a Phase III open-label safety study (M21-509).
All primary and secondary endpoints in the Phase III studies were met. The treatment demonstrated rapid onset of action as early as 8 hours after administration (the earliest timepoint evaluated), with a duration of efficacy lasting 2-3 weeks. Furthermore, the incidence of treatment-emergent adverse events with TrenibotE was similar to placebo, both as a single treatment and after up to three consecutive treatments.
