Core Medical's Corheart® 6 reaches 1,000-implant milestone, ushering in a new era of Chinese innovation in global artificial heart arena
Shenzhen Core Medical Technology Co., Ltd. ("Core Medical") recently announced that its inaugural product, the Corheart® 6 Implantable Left Ventricular Assist System, has completed its 1,000th implant. This milestone establishes it as the world's second and China's first third-generation fully magnetically levitated artificial heart to surpass the thousand-implant milestone, following Abbott's HeartMate 3 (launched in 2015).
Implant Volume Breaks Through 1,000 Cases
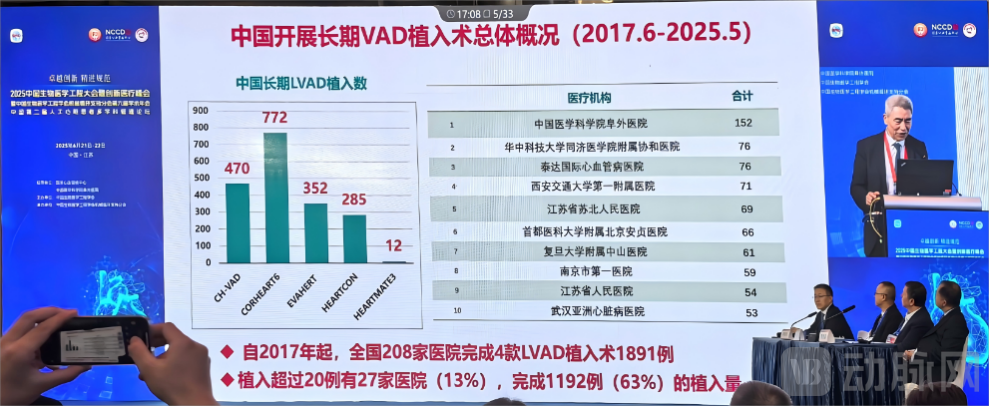
Core Medical's Corheart® 6, despite being the last domestically developed artificial heart to enter the Chinese market, has become the first to surpass the milestone of 1,000 implants. In 2025, the device has secured the leading position in China's artificial heart sector with a market share exceeding 50%. As a Chinese manufacturer of high-risk innovative medical devices, Core Medical has successfully translated its advanced R&D and technological capabilities into tangible social and economic benefits, achieving a comprehensive transition from technological breakthrough to widespread commercial application.
"Speed" and "Stability"
Core Medical has pioneered the "Time-Sharing and Zoned Dynamic Axial Fully Magnetically Levitated Control Technology" in the field of artificial hearts. The Corheart® 6, benefiting from this innovation, achieves a "simpler structure, lighter weight, smaller size, and lower power consumption." In clinical applications, the product is characterized by a "relatively small surgical incision, faster patient recovery, broader applicability across patient populations, and a reduced risk of thrombosis caused by blood pump heat." These advancements demonstrate that "China's Intelligent Manufacturing" has reached internationally leading standards in the artificial heart domain.
Throughout Core Medical's development journey, innovation-driven strategies and exceptional performance have ensured that "speed" and "steadiness" characterize every step:
In June 2021, Core Medical's inaugural product, the Corheart® 6 Implantable Left Ventricular Assist System, entered China's Green Channel for Innovative Medical Devices.
In October 2021, Corheart® 6 completed its first patient implant in China, marking the commencement of clinical applications for the world's first "axially fully magnetically levitated integrated" artificial heart product.
In June 2023, Corheart® 6 received market approval from China's National Medical Products Administration (NMPA), becoming the fourth approved artificial heart product in China.
In 2024, as a commercially available fully magnetically levitated artificial heart distinguished by its "globally smallest size, lightest weight, superior hemocompatibility, and highly portable external equipment," Corheart® 6 captured 46% of the national market share in its first launch year in China, with both market share and cumulative implant volume leading the industry.
In the international arena, Core Medical has achieved continuous breakthroughs by leveraging its proprietary magnetic levitation control technology and outstanding product capabilities. In 2024, the company completed its first overseas implant in Austria, marking the first clinical application of a high-risk active implantable medical device from a Chinese company abroad.
Technologically, through persistent exploration and innovation in integrated axial full magnetic levitation technology, Core Medical has extended its leading full magnetic levitation expertise to biventricular assist systems and infant ventricular assist devices. This expansion establishes a comprehensive product portfolio covering left ventricular to biventricular support and adult to pediatric applications.
In the same year, Core Medical's groundbreaking fully magnetically levitated integrated biventricular assist system – the world's first of its kind – entered China's Innovative Medical Devices Green Channel and completed its first clinical application in Germany.
In June 2025, at the American ASAIO 2025 conference, Core Medical presented the multicenter registry clinical trial data for its Corheart® 6 Implantable Left Ventricular Assist System. The study, which spanned 11 centers and enrolled 50 subjects, demonstrated a two-year post-implant survival rate of 86%, with the longest support duration exceeding three years. Furthermore, the application of Corheart® 6 has been extended to the pediatric field, with over 36 child implants performed to date, robustly demonstrating the global competitiveness of China's high-end medical devices.
Also in June 2025, Core Medical became the first Chinese company specializing in high-risk active implantable devices to enter the American market, completing its first commercial implant in Colombia. In July 2025, the company achieved another milestone by performing the first commercial implant of a domestically developed Chinese artificial heart in Ukraine.
From achieving over a thousand implants in China to its expanding global presence, Core Medical is steadily and rapidly consolidating its leading position in the worldwide implantable artificial heart arena. Guided by its strategy of strengthening its base in China while expanding globally, the company is writing a new chapter for China's innovative medical device industry.
Expanding the Heart Failure Treatment Portfolio
Based on publicly available information, Core Medical is concurrently advancing its portfolio of interventional artificial heart products. Its independently developed CorVad® Interventional Ventricular Assist System entered China's Innovative Medical Devices Green Channel in July 2023.
As of now, the CorVad® product series has completed multicenter clinical trials and has submitted registration applications to the National Medical Products Administration (NMPA). It is expected to address the unmet clinical need for mid- to short-term mechanical circulatory support in China, offering vital assistance to numerous patients suffering from acute heart failure and other critical cardiovascular conditions.
Unlike implantable artificial hearts, interventional artificial hearts belong to a category of circulatory support devices that can be rapidly deployed via interventional or minimally invasive techniques. They provide temporary to mid-term circulatory support for patients with acute or chronic heart failure, and can also offer intraoperative circulatory protection during high-risk cardiac surgeries. Furthermore, interventional artificial hearts can serve as a bridging therapy, assisting patients in transitioning from heart failure to eventual implantable artificial heart treatment. This therapeutic approach has been widely adopted overseas, not only enabling physicians to assess patient suitability more accurately but also significantly improving surgical outcomes and patient survival rates.
Currently, the globally commercialized and widely adopted artificial heart products primarily include Abbott's HeartMate 3 (implantable) and Johnson & Johnson's Impella series (interventional). Should the CorVad® product series receive formal market approval, Core Medical is poised to become the only company worldwide with simultaneous commercial capabilities in both implantable and interventional artificial heart solutions. The synergistic effect of these two product lines in clinical practice will further solidify Core Medical's comprehensive competitiveness and global leadership in the field of heart failure treatment.
Conclusion
Behind the thousand implants of Corheart® 6 lie a thousand stories of lives renewed—each a testament to hope and resilience. With the steady advancement of the Corheart® 6, DuoCor®, and CorVad® series, Core Medical is forging a synergistic dual-platform strategy integrating both implantable and interventional artificial heart technologies. This integrated approach will further strengthen China's heart failure treatment ecosystem and establish a comprehensive competitive advantage for Chinese enterprises in the global landscape of heart failure management.
