The new ADC competitive era: three players, one pipeline, a winning strategy
Recently, Insilico Medicine, Mabwell, and ChemExpress – three companies operating in distinct subsectors – announced a strategic collaboration. This partnership has established a novel "ABC" cooperation model (AI + BioTech/BioPharma + CXO) in the field of Antibody-Drug Conjugates (ADCs), aiming to jointly capitalize on opportunities in the ADC market.
An ADC drug consists of an antibody, a linker, and a payload. The collaboration among these three companies integrates their respective strengths: AI-driven design (Insilico Medicine), antibody platform development (Mabwell), and small molecule synthesis (ChemExpress), thereby enabling more efficient development of ADC drugs.
This synergy represents an innovation in industrial collaboration models driven by technological advancement and responds to the industry's need for technical cooperation and industrial coordination.
1Technical Cooperation Needs
The development of an ADC drug relies not on a single technology, but on the successful integration of three key elements: the antibody, the linker, and the payload. An ideal ADC drug functions like a precision-guided missile: the antibody acts as the targeting system, responsible for identifying and binding to the target; the payload serves as the warhead, tasked with eliminating the target cells; and the linker is the sophisticated safety mechanism, ensuring stability during circulation and triggering the release of the payload specifically at the disease site.
The design, development, and manufacturing of ADCs involve three technically demanding stages: large-scale antibody production, the synthesis and handling of highly potent payloads, and precise conjugation. These processes place exceptionally high demands on technical capability, specialized facilities and equipment, and quality control. This very complexity has driven the rapid growth of CXO companies specializing in the ADC field. Industry data shows that the outsourcing rate for ADC drug development exceeds 70%, significantly higher than the average 30%-40% outsourcing rate for biologics as a whole.
The success of WuXi XDC serves as a testament to the booming outsourcing landscape in the ADC sector. Data indicates that over the past five years, WuXi XDC has experienced rapid revenue growth, surging from less than RMB 1 billion in 2020 to over RMB 40 billion in 2024, with a compound annual growth rate exceeding 100%.
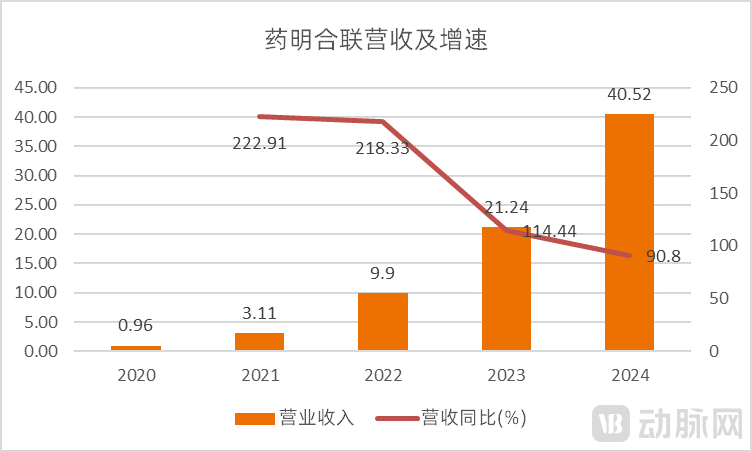
WuXi XDC's Revenue and Growth Rate Over the Past Five Years (Source: Wind, VCBeat)
Technological prowess has been a key driver behind WuXi XDC's rapid revenue expansion. The ADC field requires the integration of both macromolecule (biologics) and small molecule technologies. WuXi XDC's success is fundamentally underpinned by the talent and technological strengths of its major shareholders, WuXi Biologics in macromolecule biologics and WuXi AppTec in small-molecule chemistry, providing it with comprehensive capabilities across these two critical domains.
These capabilities are precisely what ADC drug R&D and manufacturing require. Currently, 13 of the world's top 20 pharmaceutical companies have already partnered with WuXi XDC for ADC or XDC product development. WuXi XDC provides clients with integrated CRDMO services and has now captured over 20% market share in this sector.
Partnering with WuXi XDC has become a mainstream solution for innovative biopharmaceutical companies active in the ADC field.
Now, the "ABC" model established through the collaboration of Insilico Medicine, Mabwell, and ChemExpress represents a novel approach in the ADC landscape, offering a potential solution to address the technical challenges inherent in ADC drug development.
Specifically, as a globally leading AI-driven drug discovery company, Insilico Medicine will leverage its Pharma.AI platform to design innovative payloads and linkers with novel structures and high selectivity, based on comprehensive analysis of disease mechanisms, target characteristics, and toxicological profiles. By employing AI technology, the platform can predict the drug-likeness and safety of molecules during the design phase, substantially shortening the molecular optimization process and significantly enhancing R&D efficiency.
According to Frost & Sullivan, Pharma.AI is a generative AI-powered drug discovery and development platform capable of providing end-to-end services, ranging from novel target identification to small molecule generation and clinical outcome prediction. Comprising Biology42, Chemistry42, Medicine42, and Science42, the platform covers the entire drug discovery and development process. Additionally, Pharma.AI can draft academic papers and other relevant documents, thereby enhancing the efficiency of pharmaceutical R&D.
Building upon its established expertise in molecular building blocks, ChemExpress has strategically expanded into the ADC domain. The company has developed substantial experience in the synthesis and quality analysis of payload-linker complexes, successfully establishing an integrated XDC Payload-Linker CMC platform. This comprehensive platform offers end-to-end services, including molecular design, custom synthesis, process optimization, quality studies, non-GMP/GMP manufacturing, and regulatory submission support.
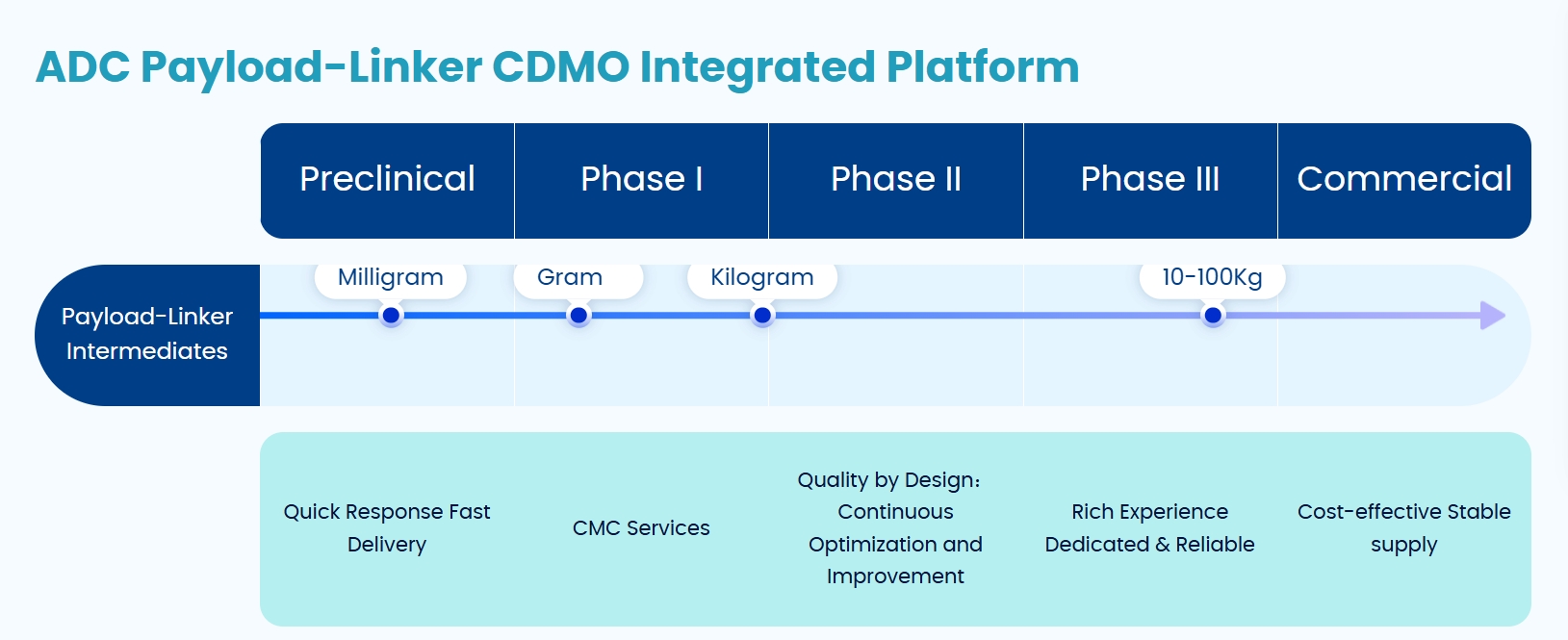
ChemExpress' ADC Payload-Linker CDMO Integrated Platform (Source: ChemExpress Website)
In terms of technical capabilities, ChemExpress currently maintains a library of over 80 stock payloads and more than 400 stock linkers, with accumulated synthesis experience covering over 1,000 linker variants. Regarding project experience, the company has provided services for ADC R&D and manufacturing projects from more than 990 clients and supported the completion of over 50 CMC projects. Notably, in the first half of this year alone, ChemExpress has undertaken more than 70 ADC projects.
ChemExpress's extensive resources in molecular building blocks and tool compounds, combined with its technical expertise and project experience in the ADC field, will provide crucial technical support for the synthesis of novel linkers and payloads in next-generation ADC drugs.
Mabwell has accumulated substantial expertise in antibody drug development over the years, with multiple monoclonal antibody products already commercialized. By collaborating with Insilico Medicine, a leader in AI technology, and ChemExpress, which possesses extensive experience in chemical synthesis, Mabwell can accelerate the development process of innovative ADC drugs.
Overall, this tripartite collaboration establishes a deeply integrated, end-to-end synergy—spanning AI empowerment, Biotech/Biopharma-driven innovation, and CRO/CDMO execution—potentially setting a new paradigm for ADC drug development.
Moreover, the advantages of this partnership extend beyond technological integration, demonstrating significant strengths in industrial coordination.
2Industrial Coordination Advantages
The development of ADC drugs requires deep integration of three distinct technological domains: small molecule chemistry, macromolecule biologics, and conjugation processes. Leveraging industrial collaboration is therefore crucial to enhancing development efficiency and optimizing drug efficacy and safety.
Notably, Insilico Medicine, Mabwell, and ChemExpress are all headquartered in Shanghai's Zhangjiang High-Tech Park, with their concentrated geographic proximity facilitating more efficient communication and enabling superior cross-industrial synergy. This collaborative advantage ensures that the three core modules—small molecules, macromolecules, and conjugation technologies—are no longer developed in isolation and simply combined at later stages. Instead, they undergo integrated design and iterative optimization from the very beginning of the development process.
Specifically, the collaboration among these three companies demonstrates several distinct advantages derived from efficient cross-industrial synergy:
1. Parallel Development and Iterative Optimization to Shorten R&D Timelines
Under the traditional linear development model, the sequential process of first developing the antibody, then screening for the payload, and finally designing the linker is not only time-consuming but also risks discovering compatibility issues at later stages. In contrast, the collaborative parallel development model enables the three core module teams to work simultaneously.
While the macromolecule team screens antibodies, they proactively consider developability factors—such as whether the antibody is suitable for conjugation and whether conjugation will impact its binding affinity—integrating these parameters into the evaluation criteria. Concurrently, as the AI-driven company designs the payload and linker, the small molecule team works in close coordination with the conjugation specialists to ensure the chemical structures are compatible with specific conjugation technologies and can be efficiently cleaved within target cells.
This parallelized and integrated approach mitigates the risk of late-stage "back-to-the-drawing-board" scenarios, significantly accelerating the identification of the preclinical candidate compound (PCC).
2. Platform-based Technology Enables "Lego-like" Rapid Innovation
Once the partners across the industrial chain have established mature technology platforms, they can subsequently generate new ADC drug candidates rapidly, in a manner analogous to building with Lego bricks.
The antibody libraries, payload-linker libraries, and validated conjugation processes from each collaborator can be seamlessly integrated and operate efficiently within the platform framework. When a novel target (such as a new tumor antigen) is identified, researchers can swiftly combine different antibodies from the library with various payload-linker combinations, enabling high-throughput screening to identify the lead candidate with the optimal activity and the widest therapeutic window. This approach significantly enhances the efficiency of early-stage drug discovery.
3. Addressing Key Challenges to Optimize the Therapeutic Window
The therapeutic window—the range between the effective dose and the toxic dose—is pivotal to ADC success, as it directly reflects the balance between drug safety and efficacy. Collaborative development provides a critical pathway to optimize this balance.
Through close collaboration, the conjugation team and the small molecule team work to optimize linker stability, preventing premature payload release before the ADC reaches the tumor site. This reduces off-target toxicity and minimizes damage to healthy tissues.
Meanwhile, by coordinating with the conjugation team, the macromolecule team can optimize the drug-to-antibody ratio (DAR). This ensures that the number of payload molecules attached to each antibody is sufficient for effective cell killing, without compromising the antibody's pharmacokinetic properties—such as causing excessively rapid clearance—thereby enhancing targeted cytotoxicity.
3Innovation Across the Entire Industrial Chain
A successful Chinese case of ADC drug innovation achieved through cross-linkage synergy is the development of RemeGen's Disitamab Vedotin (RC48).
RC48 stands as a representative example of collaborative innovation in China's ADC landscape. Its success originated from the R&D team's clinical needs-driven approach, which featured an integrated and sophisticated design of the three core ADC components—the antibody, linker, and payload—ultimately achieving highly effective synergy.
During the early stages of RC48's development, there was a significant lack of effective targeted therapies for tumors with low HER2 expression. Recognizing that an ADC drug effectively targeting HER2-low tumors would hold substantial market potential, the team focused its efforts in this direction.
The antibody utilized in RC48 is a novel HER2 antibody that has undergone humanization. This antibody exhibits a significantly higher binding affinity for the HER2 antigen compared to trastuzumab. This means that even when the number of HER2 proteins on the surface of tumor cells is low (low expression), the "targeting head" of RC48 can firmly grasp the target.
After the antibody binds to HER2, it efficiently induces membrane invagination, causing the entire ADC drug to be "engulfed" into the cell interior. This is a critical step for payload release; the higher the internalization efficiency, the greater the amount of payload delivered into the cell, and the better the cytotoxic effect.
For targets with low expression, the antibody design strategy focusing on "high affinity + potent internalization" compensates for the scarcity of target antigens, ensuring efficient intracellular delivery of the drug. The enhanced "gripping force" and internalization capability represent concrete innovations in antibody engineering.
RC48 employs the highly potent tubulin inhibitor MMAE as its payload, linked to the antibody via a cleavable linker. MMAE exhibits excellent membrane permeability. After the ADC is internalized and enzymatically cleaved within the tumor cell, releasing MMAE, the payload can not only kill the current cell but also diffuse through the cell membrane to neighboring tumor cells—regardless of their HER2 expression status.
Certainly, the collaborative innovation behind RemeGen's RC48 was achieved through the integration of internal R&D resources. In contrast, the "ABC" model pioneered by Insilico Medicine, Mabwell, and ChemExpress further consolidates resources across the entire industrial chain, thereby enhancing the potential for successful development.
The ADC field has now entered a phase of intense competition. The R&D focus of innovative pharmaceutical companies worldwide is predominantly concentrated on mature and validated targets, such as HER2, TROP2, and EGFR. Statistics indicate that the top 15 targets account for over 70% of all ADC development efforts. The popular ADC targets under investigation in China largely align with this global trend.
Amidst this highly competitive landscape, relying solely on technological advantages in a single segment is insufficient to guarantee the ultimate success of a product. Only through deep collaboration can innovative companies develop products with greater clinical value, thereby maximizing their commercial potential.
