AbbVie submits new indication application for epcoritamab in China
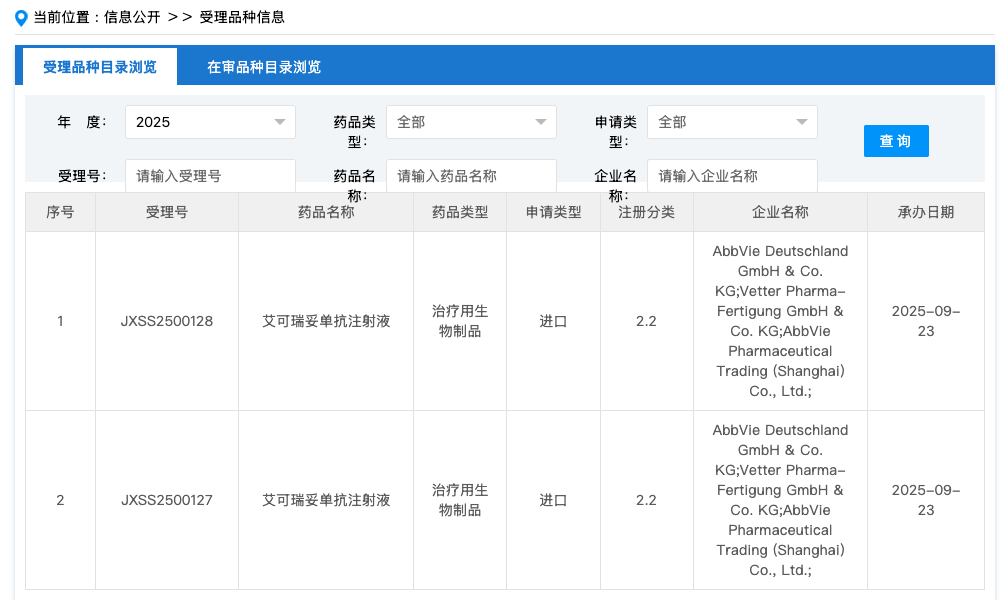
On September 23, China's Center for Drug Evaluation (CDE) website showed that AbbVie has submitted a new indication application for epcoritamab Injection for marketing approval. This new indication is for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) in combination with rituximab and lenalidomide (R2). Previously, this indication was included in the priority review by the CDE.
Epcoritamab is a CD20×CD3 bispecific antibody, originally developed by Genmab. In 2020, AbbVie entered into a collaboration with Genmab with a total value of USD 3.9 billion to jointly develop and commercialize three of Genmab's next-generation bispecific antibody products, including epcoritamab.
As an IgG1 bispecific antibody, Epcoritamab can simultaneously bind to CD3 on T cells and CD20 on B cells, and induce T cell-mediated killing of lymphoma B cells.
Epcoritamab received its first global approval in May 2023. To date, the drug has been approved for marketing in the United States, the European Union, and Japan, with approved indications covering diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and primary mediastinal large B-cell lymphoma. In 2024, the global sales of epcoritamab reached USD 281 million.
In China, AbbVie submitted the first marketing application for epcoritamab in November 2024, with the suspected indication being relapsed/refractory diffuse large B-cell lymphoma (DLBCL). The marketing application submitted this time is for the indication of epcoritamab in combination with rituximab and lenalidomide (R2) for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL).
