AstraZeneca initiates Phase III clinical trial in China for its oral small-molecule PCSK9 inhibitor AZD0780
On September 19, 2025, AstraZeneca registered the Phase III clinical trial of the oral small-molecule PCSK9 inhibitor AZD0780 on the website of China Drug Trials. This trial is intended for adults with confirmed cardiovascular disease (CVD) or adults at high risk of CVD events to reduce cardiovascular risk.
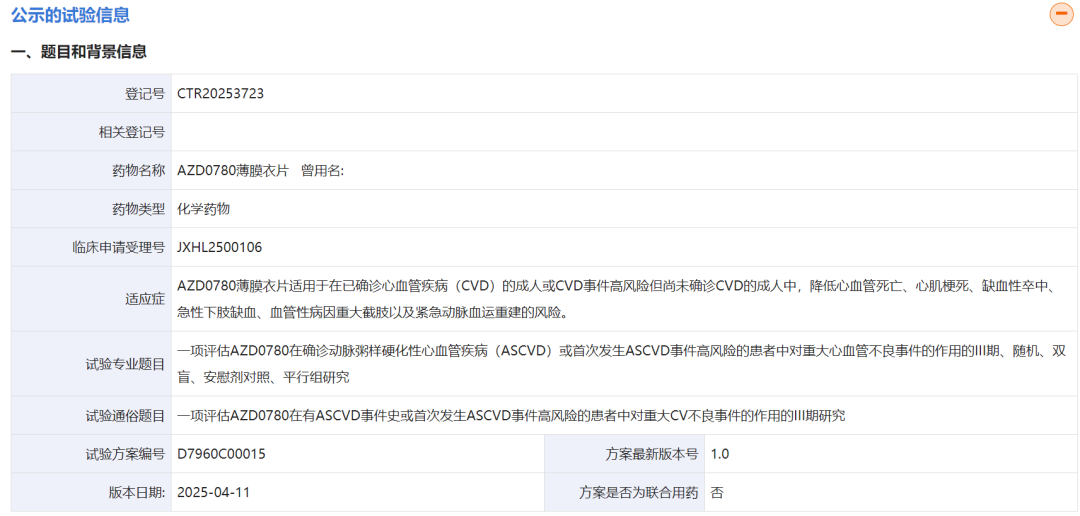
This Phase III trial in China is part of the global AZURE-Outcomes study, which launched in June with a planned enrollment of 15,100 patients and is expected to be completed by October 2029.

For this Phase III trial in China, a total of 1,195 subjects are planned to be enrolled.

AZD0780 is a small-molecule oral PCSK9 inhibitor. AstraZeneca is also considering advancing the combination use of AZD0780 with GLP-1 for weight loss and blood glucose reduction, aiming to achieve the goal of organ protection.
Source: Armstrong
