AstraZeneca's Breztri submitted for marketing approval in China
On September 17, the China's Center for Drug Evaluation (CDE) website disclosed that AstraZeneca (AZN.US) has submitted an application in China for a new indication of Budesonide/Glycopyrronium/Formoterol Inhalation Aerosol (Breztri®). Based on clinical trial progress, the proposed indication is speculated to be asthma.
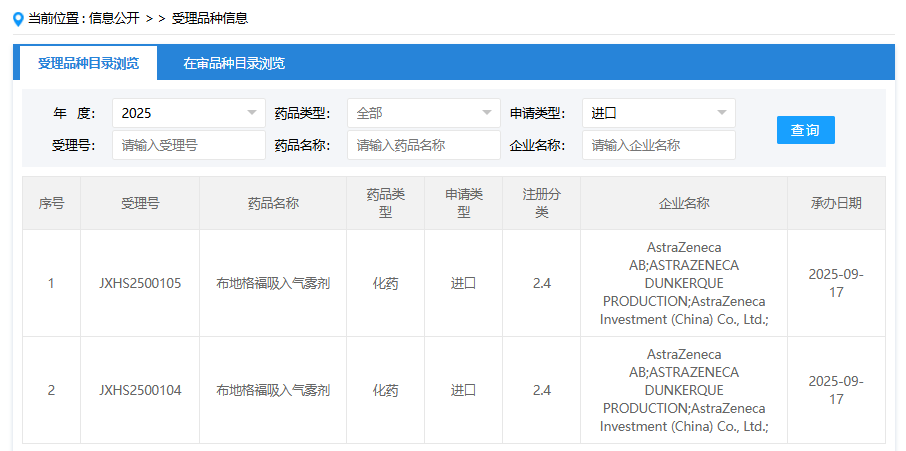
Image: CDE website
Budesonide/Glycopyrronium/Formoterol Inhalation Aerosol is a fixed-dose triple-combination therapy developed by AstraZeneca. It contains three active pharmaceutical ingredients: budesonide (an inhaled corticosteroid), glycopyrronium (a long-acting muscarinic antagonist), and formoterol (a long-acting β2-adrenergic agonist).
In May of this year, AstraZeneca announced that two Phase III studies (KALOS and LOGOS) of Budesonide/Glycopyrronium/Formoterol Inhalation Aerosol for asthma met their primary endpoints. The trials enrolled a total of 4,461 adult and adolescent patients with inadequately controlled asthma, evaluating the efficacy and safety of the triple therapy compared to the dual-combination Symbicort® (budesonide/formoterol) as maintenance treatment.
The results showed that, compared to the Symbicort® group, patients receiving Budesonide/Glycopyrronium/Formoterol Inhalation Aerosol demonstrated a significant improvement in forced expiratory volume in one second (FEV1) and a significant reduction in the rate of severe asthma exacerbations. Detailed data have not yet been disclosed.
