From rushing to rational regression: how can enterprises successfully navigate the challenges of the 2.0 era of going overseas?
When China's medical device industry secures the second spot in the global market, its expansion overseas is quietly entering the "2.0 era."
On September 15, 2025, Ruiqiao Dingke Group announced an exclusive commercialization partnership with Cosmotec Co., Ltd. (a wholly-owned subsidiary of M3, TSE code: 2413) for the Japanese market.
This strategic cooperation fully reflects the leadership advantages of Ruiqiao Dingke Group in independent research and development, compliance management, and value-driven ecological collaboration. It marks an important step in the company's global strategy and further enhances its international market influence. Meanwhile, the cooperation also highlights the advantages of Cosmotec's top-tier digital commercial platform, which can accelerate the market access of international medical devices and achieve precise alignment with clinical end-users.

Raybridge Dingke Group CEO Wang Jian and Cosmotec CEO Omi Takashi
Cosmotec Co., Ltd., established in 1992, is a wholly-owned subsidiary of Japan's M3 Group, a medical information giant under Sony. The company has built a professional clinical promotion system relying on M3 Group's online platform, providing clinical promotion channels for global medical devices. Through its digital marketing network covering 80% of licensed physicians in Japan and a cross-border medical collaboration model connecting 6.5 million healthcare professionals, Cosmotec has helped numerous international medical devices gain efficient access to the Japanese market while precisely reaching clinical endpoints.
Targeting the global medical market, this cross-border cooperation is also part of Ruiqiao Dingke Group's open commercialization strategy——"Bring in, Go global, Serve the world"An important milestone; this opportunity will further strengthen the group's connection with the physician community in Japan and even globally, deepening its..."Rooted in Asia, Serving the Globe"The in-depth layout allows advanced medical technology to transcend geographical boundaries, benefiting more patients in different countries and regions.
In the view of Wang Jian, CEO of Ruiqiao Dingke Group, "going global" is a must-answer question for China's medical device enterprises.He said, "Enterprises are like athletes. After excelling in 'domestic competitions,' they must continue to participate in high-intensity 'overseas competitions' to use competition as practice and drive their own development with higher standards."
More importantly, when going overseas is no longer just the "pass-through business" of cross-border trade, and stepping into 2.0 at the same time, the focus of going overseas is also changing quietly.Currently, mid-to-low-end domestic medical devices represented by in vitro diagnostics and low-value consumables have been mass-exported to overseas markets. However, high-value consumables with higher added value are still in the early stages of exploration for international expansion. This is because high-value consumables place a strong emphasis on clinical value, face high market access barriers, exhibit strong product usage stickiness, and their sales models differ significantly from those of mid-to-low-end products — heavily relying on clinical collaborations and a robust distributor network.
Further analysis reveals that the root of these challenges lies in gaps such as "information disparity," "channel disparity," and "trust disparity," which directly lead to high-end medical devices like domestically produced high-value consumables facing difficulties in market access and even greater challenges in scaling up during the 2.0 era of going global.
There is no doubt that how to break through barriers, take root, and rise has become the ultimate challenge that every overseas-bound enterprise must face.
Wang Jian emphasized, "Going global is an inevitable choice driven by both internal and external factors, but companies must clearly understand the essential differences between 'going global' and 'exporting'. It is crucial to focus on strengthening the three core capabilities of product strength, brand strength, and channel strength to deeply embed into the global value ecosystem."
First, widespread cultural differences, the challenges of localized operations, and the information explosion in the digital age may all contribute to overseas enterprises facing a "information asymmetry" dilemma akin to "blind men touching an elephant."
Du Xuanyuan, Head of Strategy and Business Development at Ruiqiao Dingke Group, has been involved in overseas expansion since 2010. He stated, "Overseas expansion is a protracted war. During the execution of an overseas strategy, companies need to continuously adjust and improve around strategic 'targets.' The key to establishing these 'targets' lies in eliminating information gaps regarding the local market, laws, and culture. Moreover, as long as an information gap exists, finding channels becomes particularly difficult."
From simple foreign trade exports to complex License-out, different channels require decades of local accumulation."Channel Difference"It is a key challenge that enterprises cannot avoid when going global. Although the bonus of the pandemic has allowed Chinese companies to initially establish channels and supply chains in the low-end medical device field, this is fundamentally different from channel development for "technology-oriented" high-end medical devices. More importantly, the long-standing stereotype of "Made in China" has made overseas markets price-sensitive towards mid-to-low range medical devices exported from China but lack brand trust for high-end equipment, creating a significant..."Trust Gap"。
Taken together, the convergence of these three "gaps" actually reflects deep-seated contradictions in the globalization of China's high-end medical devices: the mismatch between global market opportunities and localized capabilities, the misalignment between the pace of technological iteration and market approval cycles, and the conflict between economies of scale and the nuances of segmented markets. Together, these contradictions hinder the transition from "product export" to "value export."
Facing these systemic challenges, R-Bridge Dingke Group—a platform-based medical technology group—has actively worked to build bridges facilitating global healthcare technology exchange and progress. Through the development of three strategic engines—"independent expansion," "relay acceleration," and "strategic leap"—the group has implemented a systematic strategy to overcome key obstacles including information disparities, channel barriers, and trust gaps.
First, the "information gap" essentially reflects a "cognitive disconnect," and breaking this deadlock cannot rely solely on single approaches such as attending exhibitions or making visits.Wang Jian pointed out: "The medical device industry itself is characterized by large R&D investment, rapid technological iteration, and high regulatory thresholds. For many startups, their products may not even reach the market before the technology becomes outdated."
Ruiqiao Dingke Group's business spans three major sectors: peripheral vascular, neuroscience, and integrated oncology diagnosis and treatment. Its product range is extensive, with diverse specifications, significant clinical usage, and rapid updates, making it highly sensitive to "information gaps." In response, the group uses "capital + industry" as a link, adopting"Incubation Empowerment + M&A Acceleration + Integration Multiplier"Model, Build"High-incidence Chronic Disease Full-chain Solution Platform"。
Relying on the advantages of this "carrier-type" platform, Ruiqiao Dingke Group has formed an overseas "joint fleet" to break through the limitations of information barriers more efficiently and accurately, capture global industry trends and market demands, and achieve..."Single Product Breakthrough" to "Ecosystem Competition"The paradigm innovation of going global.
To strengthen the competitive advantage of the "Joint Fleet", Ruiqiao Dingke Group relies on the scientific research strength of the "Frontier Medical Technology Research Institute" and the "Six Major Shared Underlying Technologies" to effectively empower group companies in achieving "from 0 to 1" R&D value creation. Furthermore, with the assistance of the lean value management system of the "EBS™ Excellence System", a closed-loop value operation is constructed to effectively respond to industry changes, meet diverse needs, and ultimately realize "from 1 to N" commercial value multiplication.
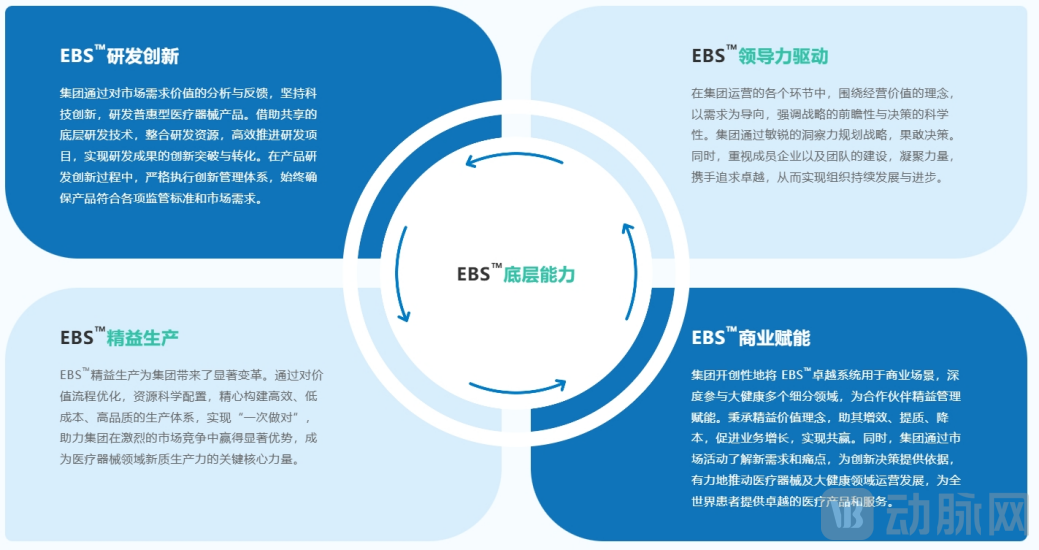
EBS™ Excellence System (EverBridge Business System)
Secondly, in breaking through the "channel gap," Raybridge Dingke Group, as a global medical technology platform enterprise based in Asia and serving the world in the field of high-incidence chronic diseases, has successfully built an international business team and overseas sales system with the support of a strong team and capital. This demonstrates its excellent global strategic network capabilities.The company not only has mature channel resources in emerging medical markets such as Southeast Asia, the Middle East, and Latin America, but also has established branches and sales outlets in countries like the United States, Brazil, and Singapore, helping overseas enterprises quickly reach different levels of global markets.
Finally, the high-end medical device track has an extremely high threshold of trust, and the establishment of trust cannot be separated from the endorsement of technical strength and the accumulation of open cooperation.In this regard, on the one hand, Ruiqiao Dingke Group has carried out innovative research combining medicine and engineering with multiple top domestic and overseas medical schools, clinical centers, and research institutions. This provides support for the innovative development and clinical application of its products while strengthening the foundation of trust through localized construction. On the other hand, international certification is not only a compliance requirement but also the cornerstone of brand trust. Through BD cooperation such as License-in/License-out with many excellent domestic and overseas medical device manufacturers, Ruiqiao Dingke further enhances its brand influence.
This collaboration with Japan's Cosmotec not only validates the "brand globalization" soft power that Ruiqiao Dingke has built in cross-cultural cooperation, laying a foundation of trust for the globalization of the group’s product lines, but will also further enhance the Japanese market's recognition and trust in Chinese brands, jointly elevating the global value of China's medical industry.
Since its establishment in 2021, Ruiqiao Dingke Group has set "internationalization" as its core strategic goal, adhering to the development philosophy of "rapid and high-quality scaling." It is committed to becoming a bridge for medical technology innovation between Asia and the world, providing innovative, high-quality, and affordable medical technology solutions for patients with chronic diseases globally, and creating a global ecosystem for chronic disease management value.
The strategic value of this "bridge ecosystem" has been fully validated in the growth paths of industry-leading companies such as United Imaging and Venus Medtech, and has also become the core advantage of Ruibridge Dingke – strong industrial resource integration capabilities.
Since its establishment four years ago, Ruiqiao Dingke has been accurately screening and integrating "high-growth, technologically complementary, and highly synergistic" companies by building an "integrated innovation platform." Leveraging the platform effect of technical synergy and resource integration, the group continuously reduces R&D and application costs, rapidly forming a closed-loop capability in "diagnosis-treatment-consumables" to jointly promote the sustainable innovation and inclusive development of medical technology.
In terms of "bringing in," Ruiqiao Dingke accelerates the landing of global innovations in China.In June 2024, an exclusive partnership with Pulmonx was established to introduce the globally leading Zephyr.®Bronchial Valve and Chartis®Pulmonary Assessment System Fills the Gap in Interventional Treatment for Severe COPD; In March 2025, the Group Partners with Japan's Kaneka Corporation to Handle Sales and Promotion of i-ED Embolization Coils in Certain Regions of China.
At the same time, the strategic pace of "going out" is also accelerating.In June 2024, Ruiqiao Dingke partnered with Vierco Medical to launch its urology products in the Latin American market. Subsequently, the group achieved several overseas milestones: receiving registration approval for its varicose vein treatment product in Uzbekistan, successfully shipping peripheral balloon products to Brazil, and obtaining registration approval for its peripheral intervention series in Turkey. In July 2025, Ruiqiao Dingke became the exclusive agent for Beijing Amaiti Medical in Latin America.
In addition, Ruiqiao Dingke has also reached strategic cooperation with Zhonghong Medical, Huanxin Medical, Guanqiao Medical, Xiangsheng Medical, and China National Pharmaceutical Group Shanghai Company, to promote the high-quality development of China's medical and health industry.
Along with the deepening advancement of the "Healthy China 2030" strategy and the continuous growth of global healthcare demands, Ruiqiao Dingke will focus on high-incidence chronic diseases, and with the support of Cosmotec, further enhance its platform framework of "rooted in Asia, serving the globe." Meanwhile, as the open strategy of "bringing in, going out, and supplying globally" continues to take effect, an accessible and affordable global healthcare innovation ecosystem will gradually take shape. As an indispensable ecological builder in the globalization of medical technology, Ruiqiao Dingke will also provide more patients with chronic diseases innovative, safe, and effective treatment solutions.
Cosmotec CEO Takashi ŌsōStated: "The product portfolio of Ruiqiao Dingke Group excels in clinical efficacy and user-centric design, perfectly aligning with Japanese doctors' pursuit of advanced treatment solutions. By combining the M3 academic platform with our localized commercial expertise, the adoption of these devices across the entire Japanese market will undoubtedly accelerate."
Wang Jian, CEO of Ruiqiao Dingke Group"Noted: 'Cosmotec's digital business model combines efficiency with accessibility, which aligns closely with our vision of promoting equitable healthcare innovation. This collaboration not only enables us to successfully navigate Japan's stringent technical regulatory environment and deliver transformative solutions to patients but also deepens global R&D synergy. Lowering the barriers to advanced healthcare through innovation and ecosystem collaboration remains a core mission of our globalization process.'"
