China's first domestic intracranial aneurysm assisted embolization stent approved for marketing; Grand Pharmaceutical Group’s “Go Global” high-end device portfolio continues to expand
Intelligent Finance APP learned that recently, the intracranial aneurysm assist occlusion stent, Blue WhaleTM of Grand Pharmaceutical Group Limited (00512), has been approved for marketing by the National Medical Products Administration (Registration Certificate No. 20253131589). It is reported that Blue WhaleTM is China's first domestically produced braided aneurysm assist occlusion stent, which not only enriches the product categories in the field of precision interventional diagnosis and treatment of cardiovascular and cerebrovascular diseases of Grand Pharmaceutical Group Limited, but also highlights the effectiveness of the company’s forward-looking layout. At the same time, it will also bring a brand-new treatment option for intracranial aneurysm treatment in China.

Domestically Produced First Braided Aneurysm Assist Occlusion Stent, Superior Performance Benefits Tens of Millions of Patients
Intracranial aneurysms, often referred to as "time bombs" in the brain, are the most common cause of cerebral hemorrhage and have an extremely high mortality rate once they rupture and bleed. Data from the "Expert Consensus on Microsurgical Treatment of Intracranial Aneurysms (2025 Edition)" shows that the overall prevalence of intracranial aneurysms in China is 1.3%-7.6%, with an approximately 7% prevalence of unruptured intracranial aneurysms in the population aged 35-75. The mortality rate within 30 days for aneurysmal subarachnoid hemorrhage caused by rupture is as high as 43%. According to Frost & Sullivan, the number of people in China with unruptured intracranial aneurysms is expected to increase to 89,514,000 by 2028.
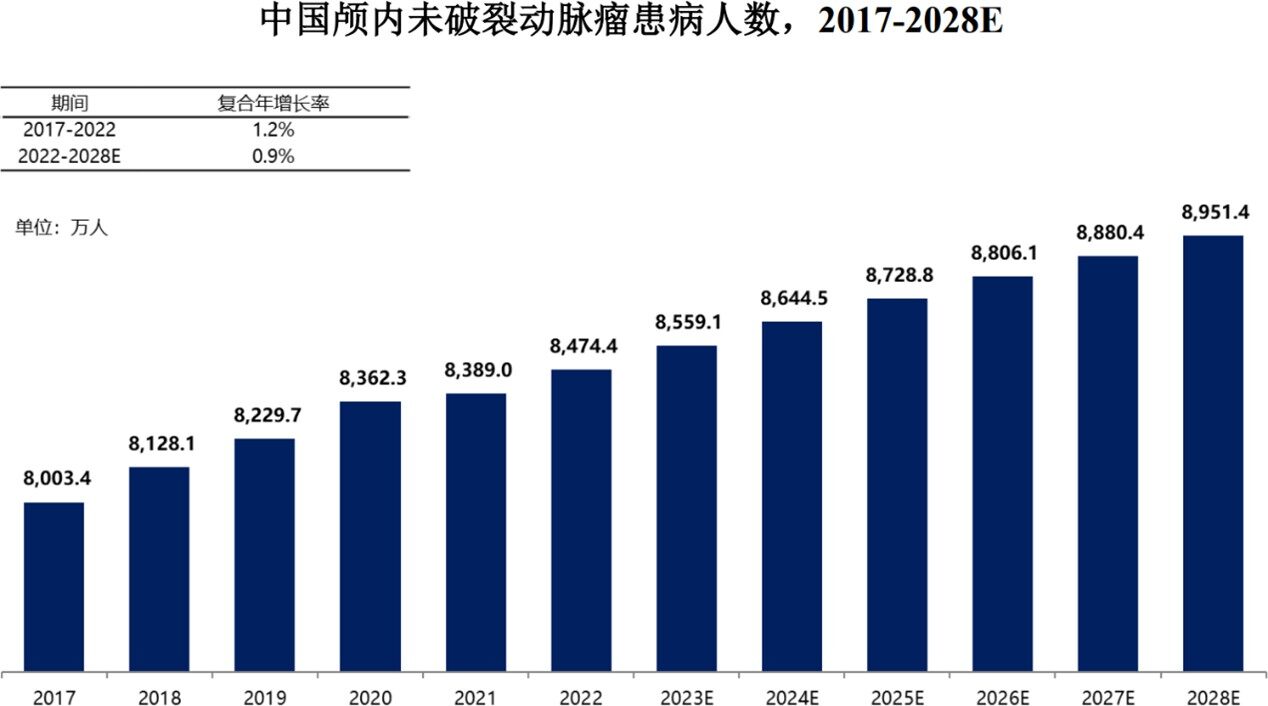
Behind the庞大的 patient numbers lies a vast and largely untapped medical market, with interventional surgeries increasingly becoming the primary choice for treating intracranial aneurysms due to their minimally invasive nature, quick recovery, and fewer complications. According to a Frost & Sullivan report, the number of interventional treatment surgeries for intracranial aneurysms in China increased from 44,000 in 2017 to 84,000 in 2022, at a compound annual growth rate (CAGR) of 13.8%. The corresponding market for medical consumables used in hemorrhagic neurointerventional treatments is expected to grow to 27.04 billion yuan by 2028, with a CAGR of 39.6%.
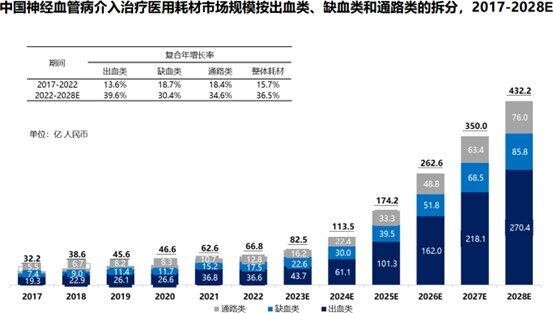
This also means that, with the popularity of interventional surgeries and the release of clinical demands, high-end medical consumables such as Grand Pharmaceutical Group Limited's Blue Whale TM will embrace a broader market space. In terms of the product itself, Blue Whale TM boasts superior performance – it is the first domestically produced braided aneurysm-assisted embolization stent, consisting of a delivery system and a self-expanding stent made of braided nitinol wires. Compared with existing auxiliary stents, its innovative structure and performance design better meet treatment needs in complex anatomical and diverse clinical scenarios.
Specifically, the Blue Whale TM adopts a unique braiding design and imaging technology to provide a certain degree of blood flow diversion, achieving over 95% angiographic aneurysm occlusion. The full series of the Blue Whale TM assist stent features a 16-wire braided design made from nitinol monofilament, significantly enhancing radial support and vessel wall apposition. Additionally, 80% of the Blue Whale TM series is retrievable, enabling controlled delivery and precise placement. With various clinical advantages such as the unique coil winding imaging technology, the Blue Whale TM intracranial aneurysm assist embolization stent is expected to provide more patients with more precise endovascular treatment options for intracranial aneurysms in the future, showing significant market potential.
Forward-Looking Layout of the Billion-Dollar Neurointerventional Device Market, Building a High-End Device Product Cluster
According to the CIC Report, the number of neurointerventional surgeries in China was approximately 161,400 in 2020 and is expected to grow at a compound annual growth rate of 28.6% to reach 740,500. With the substantial increase in the number of surgeries, the market size of neurointerventional medical devices is also continuously expanding and is projected to grow to 17.5 billion yuan by 2026.
Against this backdrop, the strategic advantages of Grand Pharmaceutical Group Limited's forward-looking layout are very significant. It is reported that Grand Pharmaceutical has been deeply involved in the field of precision interventional diagnosis and treatment for cardiovascular and cerebrovascular diseases for many years. Upholding the therapeutic concept of "precision treatment," the company has made comprehensive arrangements in three directions: pathway management, structural heart disease, and heart failure. It has built a high-end medical device product cluster. Currently, the company has arranged more than 30 products in this sector, of which 23 products have been approved for marketing in China. Other products are also actively promoting clinical registration in China. In the future, it is expected to achieve phased and echeloned launches of innovative products, driving steady growth in this sector of the company.
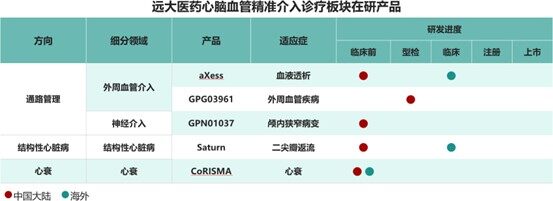
This year, the company has received regulatory approval for several innovative high-end medical devices to enter the market, with its innovative strategies gradually coming to fruition. Among these, the Platinium IberisTM Multi-Polar Renal Artery Radiofrequency Ablation System is currently the world's only Renal Denervation (RDN) product to have obtained EU CE certification and features dual access via both radial and femoral arteries. This product has completed its first post-market clinical applications. Compared with the traditional femoral artery approach, Platinium IberisTM offers significant advantages such as minimal invasiveness, faster recovery time, and the ability to perform day surgeries. Research findings related to this product have been published in full in Circulation (Impact Factor: 35.5), a leading international cardiovascular academic journal. The NeoNova® Mitral Valve Clip System, after its market launch, has quickly initiated its first commercial implants at multiple centers nationwide. Its ease of operation during surgery and postoperative outcomes have been highly praised. The domestically produced coronary and peripheral shockwave systems, DEEPQUAKE-CTM and DEEPQUAKETM, can efficiently and safely disrupt superficial and deep calcified plaques in blood vessel walls, potentially offering more diverse treatment options for patients with coronary and peripheral vascular calcification.
As an international science and technology innovation-driven pharmaceutical enterprise, Grand Pharmaceutical Group Limited is also actively expanding its global presence in the field of precision interventional diagnosis and treatment for cardiovascular and cerebrovascular diseases. The company has already established technical cooperation with clinical centers or R&D platforms in the United States, Canada, Germany, Italy, Switzerland, and other countries, and has fully built an innovative device platform that includes both “passive” and “active” technologies. In the future, with the continuous advancement of the company’s “Go Global” strategy, Grand Pharmaceutical Group Limited aims to develop this segment into a world-leading “precision interventional diagnosis and treatment platform for cardiovascular and cerebrovascular diseases,” further enhancing the company's value.
