Y-90 microsphere injection gains new European indication for unresectable intrahepatic cholangiocarcinoma and neuroendocrine tumor liver metastases, ushering in a new era in liver cancer therapy
Grand Pharmaceutical Group Limited's Nuclear Medicine "Go Global" Strategy Achieves Another Significant Progress.
According to the announcement by Grand Pharmaceutical Group Limited (0512.HK) on September 8, the company's key innovative radiopharmaceutical SIR-Spheres® Yttrium [90Y] Microspheres Injection (Y-Gan-Tai®) has recently received CE mark approval for a new indication in Europe, intended for the treatment of liver cancer patients. This approval expands the applicable scope of Yttrium [90Y] Microspheres Injection from the original indications of unresectable hepatocellular carcinoma (HCC) and unresectable colorectal cancer liver metastases (mCRC) to now include unresectable intrahepatic cholangiocarcinoma (ICC), liver metastases caused by neuroendocrine tumors (mNET), or other liver metastases, covering a more comprehensive classification of primary liver cancer and secondary liver metastases.
Notably, Grand Pharmaceutical Group Limited is actively collaborating with experts from China and abroad to develop additional indications for Yttrium [90Y] Microsphere Injection and will adopt an international registration pathway of "dual filing in China and the U.S." to promote the global market expansion of this product.
The continuous expansion of indications not only injects entirely new growth momentum into Yigantai®, but also establishes pipeline-level value with its "multi-functional single drug" characteristics, achieving therapeutic coverage equivalent to multiple product pipelines with a single product. This truly realizes the strategic breakthrough of "one product rivaling an entire pipeline."
With the significant expansion of the indications range, the number of eligible patients for Yttrium [90Y] Microsphere Injection is expected to multiply, and the market space will achieve strategic growth, ushering in a new phase of "pan-cancer treatment." At the same time, the successive overseas registration milestones also highlight Grand Pharmaceutical Group Limited's excellent overseas clinical registration and commercial operation capabilities, providing important support for the global development of the company’s subsequent self-developed innovative radiopharmaceutical products.
Unlocking High-Value Liver Cancer Indications Overseas, Doubling Global Market Potential
Yi Gan Tai® Yttrium [90Y] Microsphere Injection is the core product of the nuclear medicine anti-tumor diagnosis and treatment segment of Grand Pharmaceutical Group Limited. This product has been used in more than 150,000 patients across over 50 countries and regions worldwide. It has been recommended by several internationally authoritative treatment guidelines, including the Barcelona Clinic Liver Cancer (BCLC) guidelines, the National Comprehensive Cancer Network (NCCN), the European Society for Medical Oncology (ESMO), the European Association for the Study of the Liver (EASL), and the National Institute for Health and Care Excellence (NICE) in the UK.
After being launched in the domestic market, Yigan Tai® has continuously achieved a doubling of sales revenue, demonstrating significant market potential. By the end of 2024, the product had cumulatively treated nearly 2,000 patients and generated nearly HKD 500 million in sales revenue in 2024, with a year-on-year growth rate exceeding 140%.
While achieving remarkable commercial success, Grand Pharmaceutical Group Limited is also actively expanding the use of Yttrium [90Y] Microsphere Injection globally by unlocking more indications. In July this year, based on the breakthrough interim data from the DOORwaY90 clinical trial, the U.S. FDA granted early formal approval for a new indication of Yttrium [90Y] Microsphere Injection to treat unresectable HCC, without restricting tumor diameter size. This product has become the world’s first and only selective internal radiation therapy (SIRT) product approved by the FDA for the dual indications of unresectable HCC and colorectal cancer liver metastases. The interim results of the DOORwaY90 clinical trial showed that the objective response rate of Yttrium [90Y] Microsphere Injection in treating unresectable HCC was as high as 98.5%; all evaluable patients demonstrated a treatment response, indicating a local tumor control rate of 100%. Additionally, the median duration of response exceeded 300 days. Combined with the recent approval of a new indication in Europe, Yttrium [90Y] Microsphere Injection has gained further international recognition in the field of liver cancer treatment, while the relevant clinical data will provide critical support for the expansion of its indications domestically.
From mCRC and HCC to ICC, mNET, or other liver metastasis indications, Yigan Tai® has initially established a pipeline-level solution covering various treatment scenarios for liver tumors, significantly expanding the breadth and depth of treatments. The expansion of indications also signifies that, in the current era of rediscovered value of radiopharmaceuticals, this blockbuster radiopharmaceutical — already available in over 50 countries and regions worldwide — is showing renewed vitality. It is not only expected to enjoy a longer product lifecycle, but its global market potential could also double.
Specifically, the newly approved HCC and ICC indications will drive the Yttrium [90Y] Microsphere Injection to become a "pan-cancer treatment" product for liver cancer, a high-risk cancer.
According to GLOBOCAN 2022 data, there were approximately 870,000 new cases of liver cancer globally and about 760,000 deaths, making it the sixth most commonly diagnosed cancer and the third leading cause of cancer-related deaths worldwide. Among these, HCC and ICC are the two most common types of liver cancer cases, accounting for approximately 95% of all liver cancer patients.
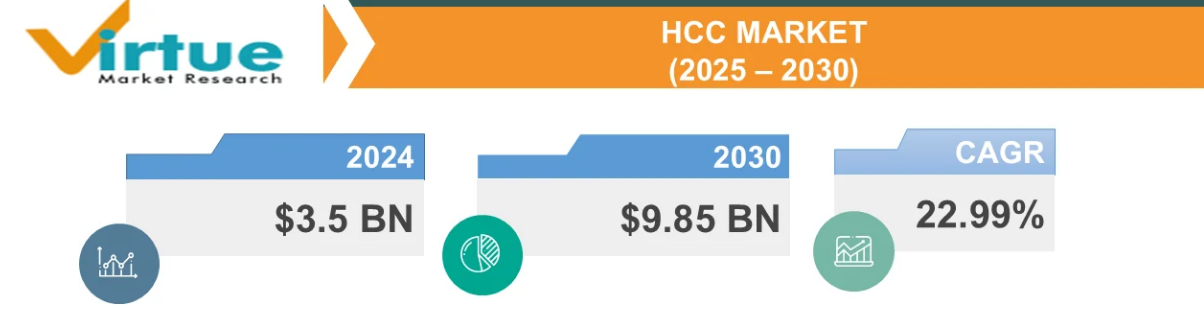
Virtue Market Research data indicates that the global HCC market size will reach 3.5 billion USD in 2024 and is expected to grow at a compound annual growth rate of approximately 23% to 9.85 billion USD by 2030, with the highest market sizes in North America and Europe.
The ICC treatment market is also considerable. According to Coherent Market Insights, the global ICC treatment market is expected to reach USD 1.1 billion by 2025 and grow at a compound annual growth rate of 8.1% to USD 1.9 billion by 2032. This also means that in the field of primary liver cancer treatment alone, the Yttrium [90Y] Microspheres Injection has already expanded the future market space by over tens of billions of dollars, with commercial potential likely to further explode.
At the same time, the expansion of indications such as secondary liver metastasis will further enhance the existing market expectations for Yttrium [90Y] Microsphere Injection. In addition to the previously approved mCRC, liver metastasis caused by neuroendocrine tumors (mNET) has also been approved as a new indication for this product. It is reported that the liver is the most common metastatic site for neuroendocrine tumors, with approximately 28%-77% of gastroenteropancreatic neuroendocrine tumor patients experiencing liver metastasis. The approval of Yttrium [90Y] Microsphere Injection for this indication will provide a new treatment option for such patients.
With the continuous increase in the penetration rate of Yttrium [90Y] Microsphere Injection in the future, its market potential in pan-hepatocellular carcinoma treatment will be further unleashed. This will not only create significant economic benefits for Grand Pharmaceutical Group Limited but also further enhance the international competitiveness of China's innovative pharmaceutical enterprises in the field of liver cancer treatment, which also highlights Grand Pharma's "Go Global" development strategy for nuclear medicine.
Globalization Development Advantages Highlighted, Full-chain Layout Builds the World's First Nuclear Medicine MNC
Seeing the big picture through a small part, the highly efficient global clinical registration of Yttrium [90Y] Microsphere Injection is a microcosm of Grand Pharmaceutical Group Limited's overseas clinical registration and commercial operation capabilities. With a global layout in nuclear medicine and an independently controlled industrial ecosystem, Grand Pharmaceutical has established an operational system covering the entire closed-loop of R&D, production, and sales worldwide. The company has built an innovative matrix in nuclear medicine that integrates diagnosis and treatment, as well as domestic and international dual circulation, continuously leading the global expansion of nuclear medicine anti-cancer therapeutic products.
Grand Pharmaceutical Group Limited has achieved a globalized nuclear medicine industry chain layout based on its R&D centers in Boston and Chengdu, four major production bases in Boston, Frankfurt, Singapore, and Chengdu, as well as a sales network covering more than 50 countries and regions worldwide.
In terms of products, Grand Pharmaceutical Group Limited currently has 15 innovative radiopharmaceuticals in the research and registration stage, covering 5 types of radionuclides including 68Ga, 177Lu, 131I, 90Y, and 89Zr, addressing 7 types of cancers such as liver cancer, prostate cancer, kidney cancer, and brain cancer. In the early research stage, the focus is mainly on RDC drugs, with a product pipeline of 12 candidates. The product categories include both diagnostic and therapeutic radiopharmaceuticals, providing patients with multiple treatment options across various indications, diverse approaches, and integrated diagnosis-treatment leading anti-cancer solutions globally. The company is one of the innovative pharmaceutical enterprises with the most comprehensive product pipelines and integrated diagnosis-treatment layouts in the field of radiopharmaceutical oncology worldwide. It also holds the largest total reserve of diagnostic and therapeutic RDC innovative drugs entering Phase III clinical trials in China.
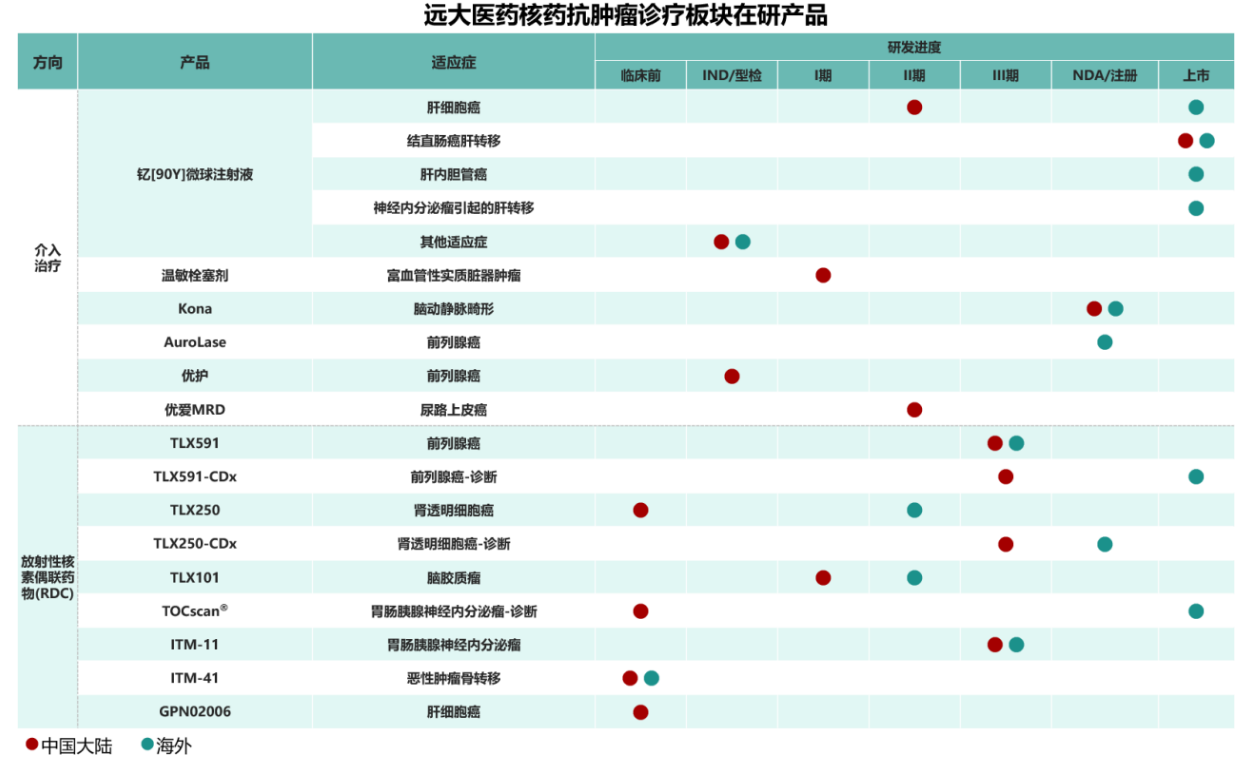
In terms of R&D, Grand Pharmaceutical Group Limited is actively promoting the global clinical research of innovative radiopharmaceuticals. In 2025, the company's self-developed innovative radiopharmaceutical GPN02006 has achieved breakthrough clinical progress and was selected for an oral presentation at an international authoritative conference, with the potential to become the world’s first RDC product for HCC diagnosis targeting the GPC-3 site. Additionally, innovative products such as TLX591 and ITM-11 have been included in international multicenter Phase III clinical trials, fully demonstrating the company’s operational strength in global clinical research.
In terms of global supply chain construction, the company's Chengdu radiopharmaceuticals R&D and production base is equipped with 14 high-standard GMP production lines, capable of independently producing various isotopes. It is one of the smart factories with the most comprehensive range of radionuclides and the highest degree of automation globally, as well as the world’s first closed-loop platform for the entire nuclear medicine industry chain. In the future, this base is expected to accelerate the implementation of Grand Pharmaceutical Group Limited's global innovative R&D pipeline, promote high-quality development of the nuclear medicine industry, cultivate high-value blockbuster products, and lay a solid foundation for the domestic production of the company’s radiopharmaceuticals.
With its leading product technology, solid clinical evidence, efficient registration capabilities, and a comprehensive global supply chain system, Grand Pharmaceutical Group Limited has secured a leading position in the global nuclear medicine race. The company stated that in the future, it will leverage its world-leading nuclear medicine platform and industrial chain advantages to actively develop innovative radiopharmaceuticals aimed at addressing key challenges in oncology diagnosis and treatment. At the same time, the company will continue to adhere to its strategy of independent research and innovation in nuclear medicine as well as the "dual filing in China and the U.S." approach, steadily advancing international multicenter clinical trials to expand into global markets. This effort aims to promote the high-quality development path of "Go Global" for the radiopharmaceuticals industry and establish itself as the world's leading nuclear medicine multinational corporation (MNC).
