Approved innovative device, China's first transcatheter aortic valve via vascular access
Recently,GenesisAnnounces Its SubsidiarySuzhou Jiecheng Medical Technology Co., Ltd.(Abbreviation:Jiecheng Medical")independently developed by "J-VALVE®TF Transcatheter Aortic Valve System"Approved for NMPA Innovative Product Registration (Registration No.: 20253131748)."
This innovative breakthrough approval will effectively change the dilemma of elderly severe AR patients having "no minimally invasive devices available," becoming another milestone in the field of interventional treatment devices for structural heart disease in China, bringing new hope to more critically ill patients.
# From Scratch: Filling the Domestic GapARInterventional Therapy Gap
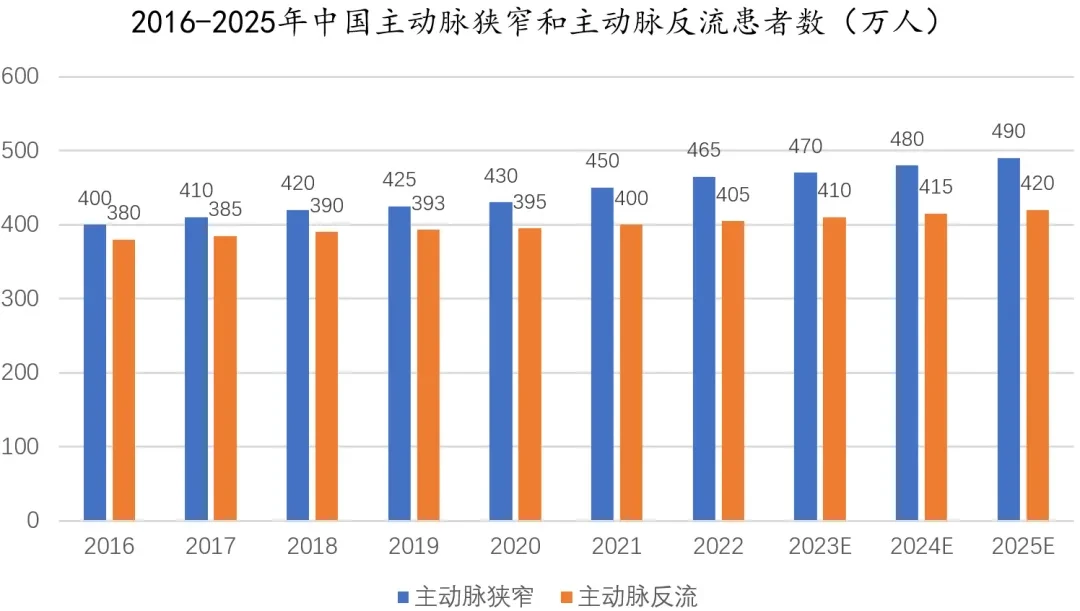
For a long time,ARPatients face significant limitations in treatment options. While surgical procedures are well-established, the surgical risks are extremely high for elderly patients with multiple comorbidities.The lack of targeted interventional therapeutic devices worldwide has led to a large number of critically ill patients.ARPatients can only rely on medication for symptomatic support and are unable to receive effective interventional treatment.
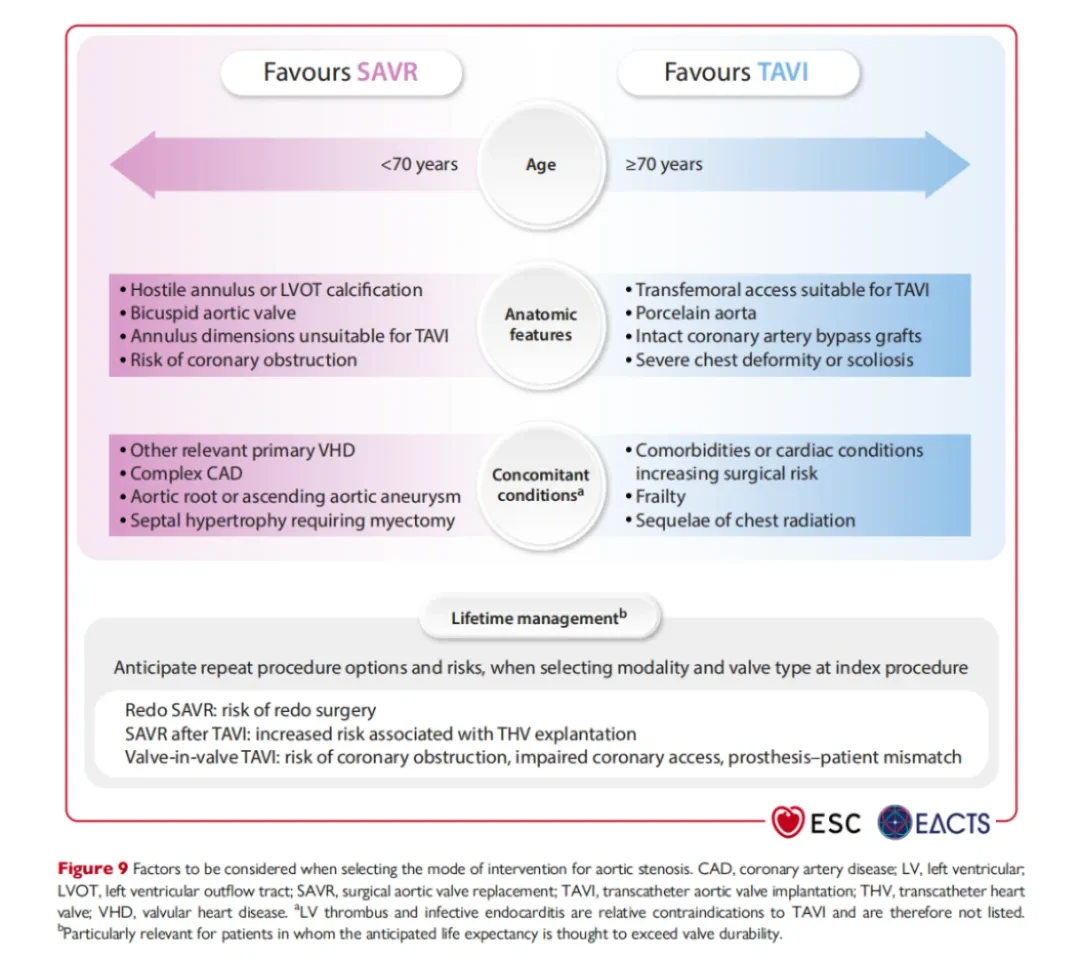
Recently,European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Jointly released the much-anticipated "2025 Valvular Heart Disease (VHD) Management Guidelines"It was pointed out that,For AR, the guidelines also keep pace with the times,Proposed for the first timeFor patients with symptomatic severe conditions who are not suitable for surgeryAR Patients, if the anatomical conditions are suitable, may consider undergoingTAVI Treatment(IIb,B Recommendation).
J-VALVE® TF The advent of this device is an original solution targeting this long-standing clinical pain point. It not only enables our country to achieve“From Zero to One”A leap forward, and alsoFor the first time,“Stenosis+Incomplete Closure”The treatment pathways for dual indications are fully established.This means that Chinese doctors will have a more comprehensive arsenal of interventional treatments when facing patients with complex valve diseases, providing greater scope for precision medicine.
# 3Genesis:BuildARTherapeutic“China Solution”
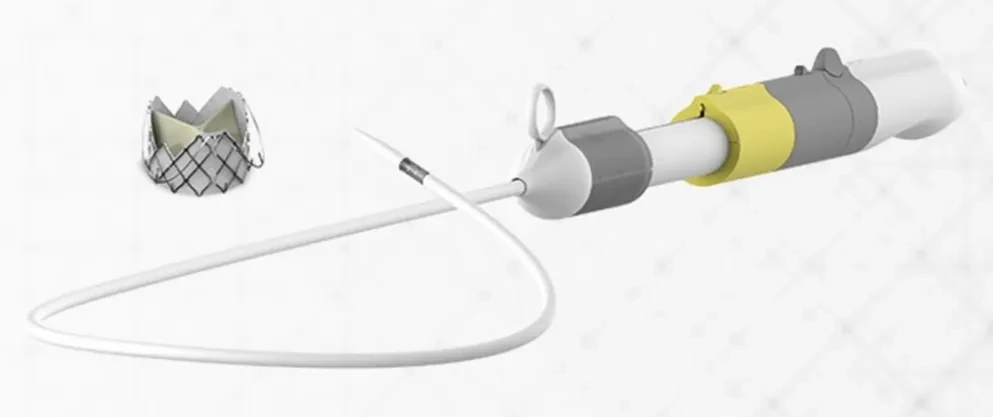
J-VALVE® TFTranscatheter Aortic Valve SystemForThe First in ChinaObtainNMPAApproved, Transcatheter Aortic Valve Products for Treating Aortic Regurgitation via Vascular Access,Femoral Artery Access, with a movable positioning piece clamping the native aortic valve leaflets, and a bendable delivery system adaptable to complex anatomical structures such as horizontal hearts. Suitable for patients assessed by the cardiac team in conjunction with a scoring system as having symptomatic, severe aortic valve insufficiency (severe aortic regurgitation), or combined with aortic stenosis, who are not suitable for conventional surgical valve replacement, and are aged greater than or equal to70-year-old patient.
The product consists of a transcatheter bioprosthetic valve and a transcatheter delivery system. Through minimally invasive interventional technology, it enables valve replacement without the need for open-heart surgery, significantly reducing surgical risks and providing a new treatment option for elderly patients or those unable to tolerate traditional surgical procedures.
At the product innovation level,J-VALVE® TF By“Three Major Groundbreaking Innovations”As the core highlight, it accurately addressesARThe Most Challenging Problem in Treatment.
1. Domestically First Innovative Movable Leaflet Positioning Technology
TargetingARThe problem of the patient's valve leaflets being weak and difficult to position,J-VALVE® TF Innovatively introduced“Movable Leaflet Clamping and Positioning”Technology. This design elevates the precision and stability of valve positioning to new heights through a unique dynamic clamping structure, effectively preventing positioning shifts caused by insufficient leaflet support. This not only significantly reduces the complexity of surgical procedures but also greatly enhances the safety of the surgery.
2. Implanted via the femoral artery route to reduce trauma risk
Traditional interventional valve implantation often requires open-chest surgery or a small incision through the apex, which poses significant risks for elderly patients and those with comorbidities.J-VALVE® TF Innovatively adopts the femoral artery approachThe implantation procedure is completed without the need for open-chest surgery, and can be achieved solely through minimally invasive puncture. This breakthrough design not only reduces operation time but also significantly improves postoperative recovery speed and comfort, greatly enhancing the benefits for patients.
3. Adjustable Bend Delivery System, Covering a Wider Range of Anatomical Types
Patients with aortic regurgitation often present with complex cardiac anatomical features, such as horizontal heart, aortic root deformities, etc.J-VALVE® TF Equipped withComplex Anatomy Adaptive Adjustable Bend Delivery System, capable of flexibly handling various special anatomical situations, covering more than85%OfARPatients. This means that its clinical applicability is far broader than previous interventional products.
Not only that, but also focusing on the above core breakthroughs,J-VALVE® TF A systematic layout has been formed at the intellectual property level. With“Movable Leaflet Clamping and Positioning”Technology, the company has established a multi-dimensional coverage including structural design, drive mechanism, sealing technology, material improvement, and delivery system optimization.12Core Invention Patent Cluster, and completed in Europe, the United States, Japan, etc.18The patent layout in various countries and regions. This not only lays the foundation for its international promotion but also demonstrates the foresight and strategic positioning of China's original medical devices in global competition.
# Solid Clinical Data:Dual Validation of Safety and Efficacy

J-VALVE® TF The approval was not accidental but based on solid clinical data. Its prospective multicenter clinical trial was conducted byProfessor Wang Chunsheng from Zhongshan Hospital, Fudan UniversityLed by, a total of nationwide18Home Center127Example: Moderate to Severe and SevereARPatient.
1. Implant Success Rate and Operational Feasibility
In127Among the patients, there are124ExampleSuccessfully CompletedJ-VALVE® TF Implantation, with a success rate as high as97.6%。Only3Example of conversion to surgical aortic valve replacement due to anatomical or procedural factors (SAVR). This fully illustrates thatJ-VALVE® TF The high feasibility in operation provides a replicable experience for cardiovascular intervention doctors in clinical practice.
2. Follow-up Results Show Long-term Stability
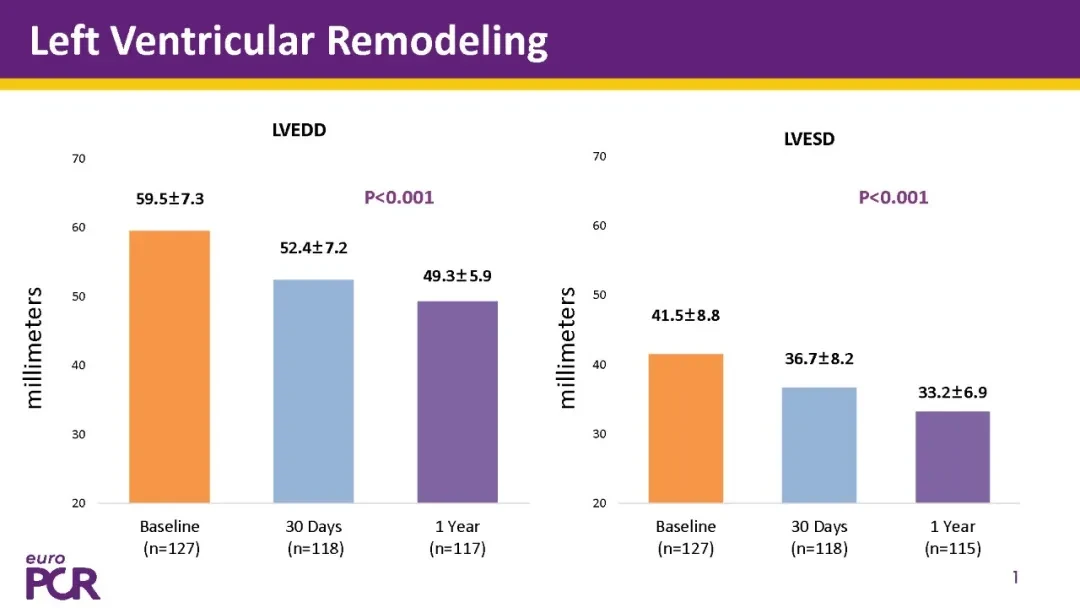
The study was conducted on patients30Day,6Months,1Year and5YearFollow-up at multiple time points.1The primary endpoint of the annual follow-up was all-cause mortality, while the secondary endpoints covered multiple dimensions including cardiovascular mortality, permanent pacemaker implantation rate, valve hemodynamic performance, left ventricular remodeling, cardiac function improvement, and quality of life.
Results:
Low all-cause mortality:Postoperative12The cumulative all-cause mortality rate over the months was significantly lower than the risk level of traditional surgical treatment.
Significant Improvement in Cardiac Function:The patient's left ventricular remodeling is significant,NYHA Cardiac function classification generally improved.
Low pacemaker dependency rate:The low implantation rate of permanent pacemakers indicates that this product has a good advantage in protecting the cardiac conduction system.
Valve function stability: PlantThe valve's opening and closing function is good, with excellent hemodynamic performance, and no significant regurgitation or stenosis was observed.
Quality of Life Improvement:Most patients experienced significant relief from heart failure symptoms after the surgery, with substantial improvements in exercise tolerance and quality of life scores.
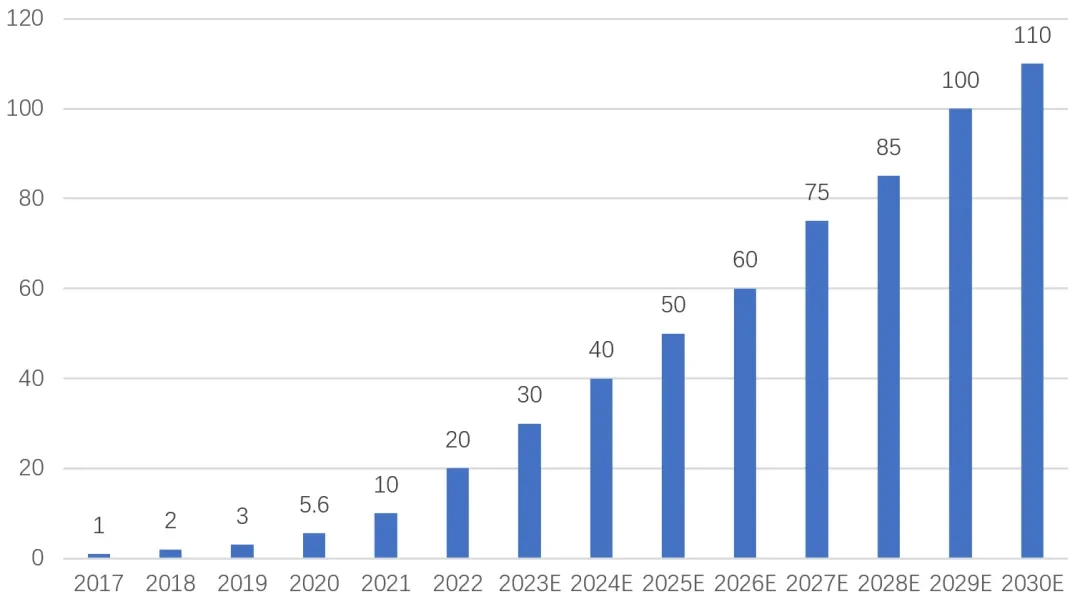
These results not only demonstrate thatJ-VALVE TF The significant value in therapeutic efficacy further validates its long-term safety and sustainable clinical benefits. Especially given the global shortage ofARAgainst the backdrop of specialized interventional products, these data highlight their“Global Leader”The meaning.
# From Breaking the Stalemate to Taking the Lead: The Growth Trajectory of the Product
1. First-generation ProductJ-VALVE TA: Break the import monopoly
2017Year, the first-generation product developed by Jiecheng MedicalJ-VALVE TA Transcatheter Aortic Valve Replacement via Apical ApproachNMPAApproved for marketing. It is not only in the field of domestic interventional valves“Icebreaker”, and has also become one of the few companies globally to simultaneously coverASAndARA product with dual indications. However, due to its use of the transapical mini-incision approach, it still has limitations such as larger trauma and longer recovery period.
2. Genesis Medical Promotes Second-Generation Upgrade:J-VALVE® TF
As the parent company of Jiecheng Medical, Genesis continues to invest heavily in product iteration.2023Year,J-VALVE TF Successfully completed domestic registration clinical enrollment and obtained in the same year in the United StatesFDA“Breakthrough Medical Device Designation”。FDA The reasons for approval point directly to two aspects:
Globally unique design of movable positioning components;
Fill the internationalARA Major Technological Gap in Interventional Therapy.
This milestone event has enabledJ-VALVE® TF Becoming one of the few domestic entities to enterFDA“Breakthrough Channel”The cardiovascular interventional devices have laid a solid foundation for internationalization.
3. Dual-track Parallel: Domestic and International Clinical Validation
2024Year,J-VALVE® TF The domestic production and internationalization process are accelerating simultaneously. In the domestic market, the product has entered the follow-up and data accumulation phase, which willNMPAApproval provides critical support; internationally, early feasibility studies (EFS) Completed enrollment, initiated pivotal clinical trials, and obtainedFDAFurther approvals demonstrate strong global competitiveness.
4. Clinical Data Summit: Final Approval Obtained
2025Year,J-VALVE® TF Clinical Data Debuts on Multiple International Stages:
CHINA VALVEConference:6The follow-up results at X months showed that the all-cause mortality rate was only2.36%, Permanent Pacemaker Implantation Rate10.24%, all core indicators have reached internationally advanced levels.
EuroPCRConference:12The follow-up results at X months showed that the all-cause mortality rate was only3.2%,Ⅲ°The incidence of atrioventricular block is as high as94.5%, Refreshing Global PurityARThe clinical records of interventional treatment have gained high recognition in the international academic community.
With this series of solid results,J-VALVE® TF Finally, in2025Year9Monthly AcquisitionNMPAApproval, completing the critical leap from R&D to market launch.


Suzhou Jiecheng Medical Technology Co., Ltd.(Suzhou Jiecheng Medical Technology Co., Ltd., abbreviated as "Jiecheng Medical") was established in September 2009. It is a high-tech enterprise specializing in the research, development, and manufacturing of advanced cardiovascular medical devices.Holding multiple invention patents, the leading project isInnovative "J-Valve Precision Positioning Heart Valve Implantation System"Technology research and development and product production. In 2022, Suzhou Jiecheng Medical Technology Co., Ltd. officially joined Genesis.
