Domestically-produced first-of-its-kind, invasive brain-computer interface Class III medical device approved for market launch
Recently,Nanjing Shanhai Medical Technology Co., Ltd.'s self-developed "Functional Neurosurgical Electrophysiological Recording and Stimulation Device" (Trade Name: Mingtong) has officially received approval from the National Medical Products Administration (NMPA) for market launch., becoming the first domestically approved Brain-Computer Interface Class III medical device and the first domestically produced invasive deep-brain electrophysiological recording and stimulation device. This equipment marks a key technological breakthrough in the field of invasive brain-computer interface medical devices in China.
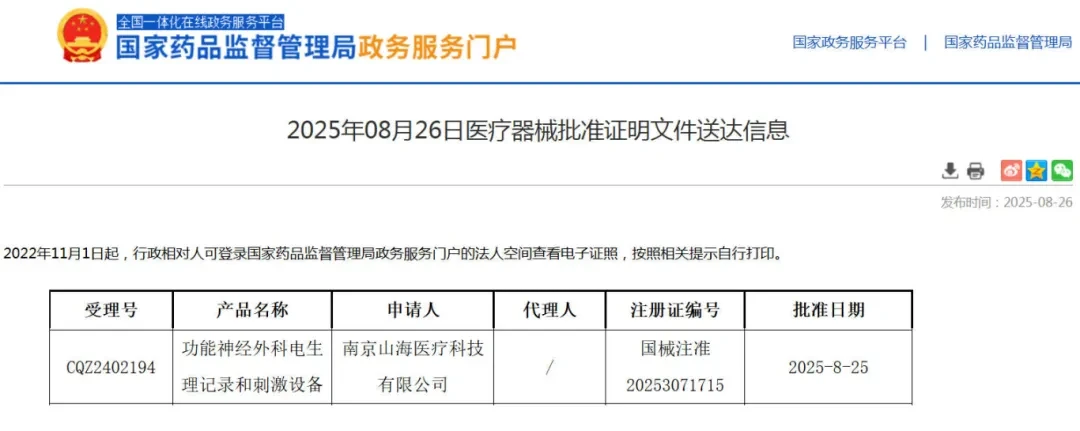
1. The First Domestic Invasive Brain-Computer Interface Class III Medical Device
"Mingtong" as a high-end electrophysiological device dedicated to functional neurosurgery, its core application lies in the real-time acquisition, localization, and regulation of neuroelectrophysiological signals during deep brain stimulation (DBS) surgery. Its technological highlights include:

High-Precision Signal Acquisition: Supports high-resolution acquisition of multi-channel electrophysiological signals from deep brain nuclei, capable of accurately capturing microvolt-level neural signal changes. Real-time Neural Navigation: Integrating real-time signal analysis algorithms with a three-dimensional neural navigation system to assist doctors in dynamically adjusting the electrode implantation path during surgery, improving positioning accuracy; Multimodal Stimulation Regulation: Supports dynamic adjustment of electrical stimulation parameters, enables simultaneous signal recording and neuromodulation, optimizing surgical efficacy and safety. This device successfully breaks through foreign technical monopoly, realizing the domestic substitution of the DBS intraoperative neurophysiological recording system, and fills the gap in the domestic field of invasive deep brain electrophysiological devices.
Brain-Computer Interface (BCI), as an interdisciplinary frontier field integrating neuroscience, engineering, and clinical medicine, has demonstrated clinical application potential in the treatment of Parkinson's disease, epilepsy monitoring, and paralysis rehabilitation. The approval of "Mingtong" not only verifies the safety and efficacy of domestically produced invasive BCI devices but also establishes a complete chain from technical development to clinical translation, providing a technical paradigm and clinical validation platform for the subsequent development of BCI-related medical devices.

2 Company Background and R&D Strength
Nanjing Shanhai Medical Technology Co., Ltd., as a national high-tech enterprise, focuses on product solutions for brain diseases and brain science, establishing a full-chain R&D capability from sensor design, chip development to signal processing algorithms. The company collaborates deeply with top domestic medical institutions and universities, forming a "industry-university-research-medicine" collaborative innovation model, continuously promoting the R&D and implementation of neuro-electrophysiological technology platforms, innovative neuromodulation devices, and digital therapeutic tools.
The approval of "Mingtong" marks an important milestone in the R&D journey of Mountain-Sea Healthcare and is a crucial step toward accelerating the development of an independently controlled digital diagnosis and treatment ecosystem for brain diseases. In the future, the company plans to further expand the clinical application of BCI technology in areas such as neurodegenerative diseases and mental illnesses through an efficient closed loop between research, clinical practice, and industry.
The launch of "Mingtong" marks China's entry into the international advanced ranks in the field of invasive brain-computer interface medical equipment. Its technological breakthroughs and clinical value will provide biomedical professionals with new diagnostic and therapeutic tools, while setting a benchmark for the research, development, and translation of high-end domestic medical devices.
