Global First ADC Drug - Tisotumab Approved in Hong Kong, Reduces Cervical Cancer Mortality Risk by 30%
On September 3, 2025, according to an announcement by Zai Lab, Inc.,Tisotumab(Tisotumab vedotin-tftv, Trade name: Tivdak®)Approved by the Hong Kong Department of Health, for the treatment ofAdult patients with recurrent or metastatic cervical cancer that has progressed during or after chemotherapy。
The approval was based on the positive data from the innovaTV301 trial: the median overall survival for patients treated with tisotumab was 11.5 months, significantly longer than the 9.5 months in the chemotherapy group, with a 30% reduction in the risk of death. This breakthrough marks a major advancement in the field of cervical cancer treatment.

▲Screenshot source“OncLive”
Tisotumab vedotin-tftv (brand name: Tivdak) is a tissue factor (TF)-targeted antibody-drug conjugate (ADC).
On April 29, 2024, the drug was approved for marketing by the U.S. Food and Drug Administration (FDA) based on the results of the innovaTV301 trial for the treatment of adult patients with recurrent or metastatic cervical cancer who progress during or after chemotherapy—making it theThe first and currently only ADC drug approved for the treatment of cervical cancer。
In March 2025, Japan's Ministry of Health, Labour and Welfare approved tisotumab for the same indication, marking the first ADC drug approved in Japan for the treatment of cervical cancer; in April of the same year, the European Commission also approved the drug as a monotherapy for treating such patients.
On September 3, 2025, the long-awaited news finally came that the drug was approved for marketing in China (Hong Kong), bringing new hope to patients with recurrent or metastatic cervical cancer who have limited treatment options!
The approval of tisotumab vedotin is primarily based on positive data from the phase 3 clinical trial innovaTV301 (NCT04697628). The study enrolled a total of 502 patients with recurrent cervical cancer, who were randomly assigned to two groups: the tisotumab vedotin group (253 patients received tisotumab vedotin monotherapy) and the chemotherapy group (249 patients received treatment with topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed). The results are as follows:
1、Objective Response Rate (ORR): Confirmed in the Tisotumab groupORR reached 17.8%(95%CI:13.3%-23.1%),Much higher than the chemotherapy group's 5.2%(95%CI:2.8%-8.8%)(P<0.0001)。
2、Median Overall Survival (OS): Tislelizumab groupThe median OS was 11.5 months, significantly longer than 9.5 months in the chemotherapy group.,Equivalent to a 30% reduction in mortality risk(Hazard ratio 0.70; 95% CI: 0.54-0.89; P=0.0038).
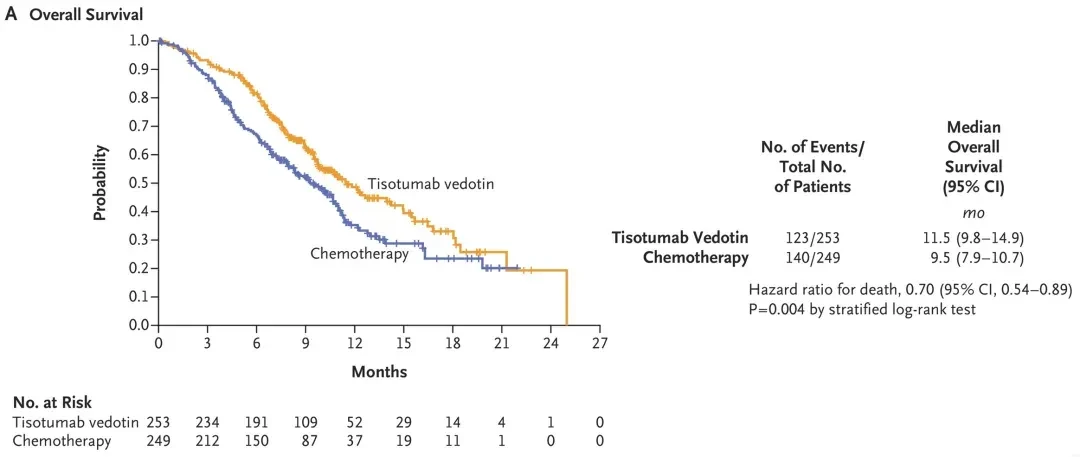
▲Image Source"N Engl J Med", owned by the original author. If we unintentionally infringe on intellectual property rights, please contact us for removal.
3、Median Progression-Free Survival (PFS): Tisagenlecleucel GroupMedian PFS was 4.2 months(95%CI:4.0-4.4),2.9 months longer than the chemotherapy group(95% CI: 2.6-3.1) (Hazard Ratio 0.67; 95% CI: 0.54-0.82; P<0.0001).
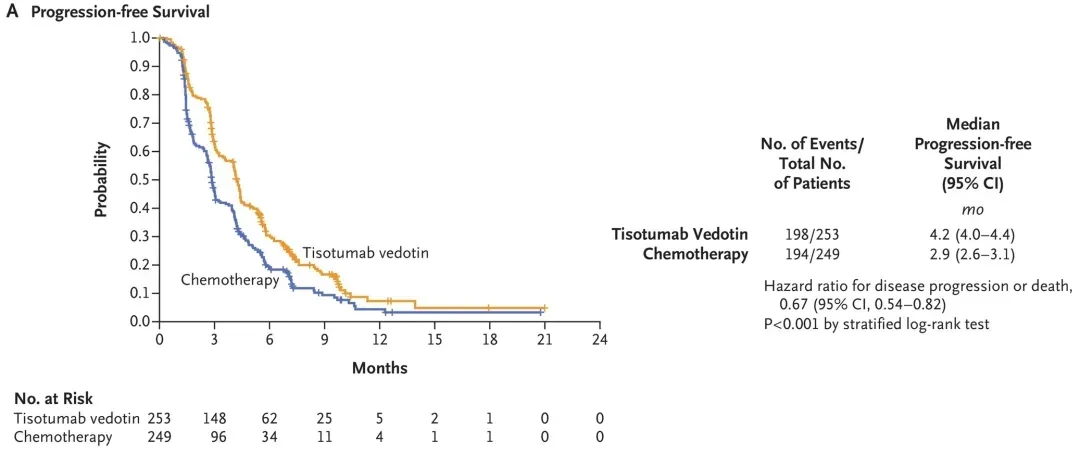
▲Image Source"N Engl J Med", the copyright belongs to the original author. If we unintentionally infringe on intellectual property rights, please contact us for removal.
In addition to the aforementioned Tisotumab, there are numerous new drugs and technologies for cervical cancer being actively researched in our country, bringing new hope and more effective treatment options to cancer patients. We also look forward to these new drugs passing review smoothly and being applied in clinical practice as soon as possible, so that more cancer patients can have access to effective treatments!
The good news is that several investigational drugs have successively entered the clinical trial stage, which also means that Chinese patients will have the opportunity to receive free treatment with the latest anti-cancer drugs. At the same time, the "Ark Aid Program" can also provide patients with the opportunity for free treatment with approved or unapproved new anti-cancer drugs. Patients who are dissatisfied with existing treatment options or have developed drug resistance can compile and submit their recent pathology and imaging examination reports, treatment history, and other relevant materials toGlobal Oncology Doctors Network Medical Department (400-666-7998), conduct a preliminary assessment or understand the detailed inclusion and exclusion criteria.
[1]Vergote I,et al.Tisotumab vedotin as second-or third-line therapy for recurrent cervical cancer[J]. New England Journal of Medicine, 2024, 391(1): 44-55.
https://www.nejm.org/doi/full/10.1056/NEJMoa2313811
[2]https://www.onclive.com/view/tisotumab-vedotin-approved-in-hong-kong-for-recurrent-or-metastatic-cervical-cancer

