100 Million Yuan Upfront Payment! Domestic Bispecific ADC Goes Global
August 29, 2025, Keyi (Zhejiang) Pharmaceutical Technology Co., Ltd. (hereinafter referred to as Keyi Pharma) announced its global first nano bispecific antibody-drug conjugateExclusive Overseas Development, Registration, and Commercialization Rights of KY-0301 Granted to Radiance Biopharma(hereinafter referred to as Radiance),Total transaction consideration up to $1.165 billion, including a $15 million upfront payment, up to $150 million in R&D/registration milestones, and up to $1 billion in commercialization milestones, along with sales-based royalties.
From Nano Bispecific Antibody to Uniform DAR: KY-0301 Achieves Dual Filing and Dual Approval
Classic bispecific ADCs can improve targeting accuracy and resistance to drug resistance through dual targets,But often constrained by three major bottlenecks: insufficient tissue penetration efficiency (traditional antibody molecular weight is about 150kDa, making it difficult to penetrate solid tumors), narrow toxicity window (non-specific release of cytotoxic drugs), and drug-to-antibody ratio (DAR) uniformity (low product purity leading to batch-to-batch variability).。
KY-0301 adopts a bispecific binding arm configuration with 12-15kDa nanobodies (Nanobody) and is conjugated and optimized using Keyi's self-developed TPEBEN platform. According to publicly available data, it shows significant improvements in tissue penetration, delivery efficiency, and safety compared to traditional ADCs.At the process level, its DAR4 structure has achieved a purity of approximately 98%., which helps improve the controllability and batch-to-batch consistency in predicting dose-exposure-toxicity relationships, thereby providing a more reliable basis for safety evaluation in Phase I clinical trials.
More crucially,KY-0301 simultaneously targets c-MET (mesenchymal-epithelial transition factor) and EGFR (epidermal growth factor receptor).These two targets are widely expressed in various tumors (such as non-small cell lung cancer, colorectal cancer, and squamous cell carcinoma of the head and neck), which is the logical starting point for their exploration in major indications like non-small cell lung cancer, colorectal cancer, and squamous cell carcinoma of the head and neck.
On the regulatory pathway,KY-0301 received FDA clinical trial approval in December 2024 and obtained implied permission from China's National Medical Products Administration (NMPA) in January 2025,Achieve dual filing and dual approval in China and the USCurrently, KY-0301 has initiated a Phase I/II multicenter clinical trial (NCT06928363) in China, entering the dose escalation and expansion phase to evaluate its safety and preliminary efficacy in patients with various advanced solid tumors.
For Keyi (Zhejiang) Pharmaceutical Technology Co., Ltd., this authorization is not just a consideration, but also an accelerator that transforms technological potential into global experimental potential.
For Radiance, the selling point of KY-0301 lies in its transformation potential: nano bispecific antibodies offer deeper penetration into the tumor microenvironment and better coverage of heterogeneity/resistance; the design of uniform DAR and safety enhances controllability in early clinical trials, thereby reducing operational risks in advancing international multicenter trials; dual submission and approval along with initiating domestic multicenter Phase I/II trials means rapid supplementation of dose-safety data for key populations, creating room for growth for bridging studies or parallel global multi-regional trials.
Keyi (Zhejiang) Pharmaceutical Technology Co., Ltd.: "Platform + Pipeline" Dual-Driven Strategy for Precision Delivery
Keyi (Zhejiang) Pharmaceutical Technology Co., Ltd. was founded in 2018, focusing on innovation and clinical translation in tumor immunotherapy., and holds a controlling stake in Shanghai Keyi Pharmaceutical. The company has a clear layout: one is the bispecific antibody platform, two is the nano bispecific ADC platform (TPEBEN), and three is the enhanced dual-target CAR-T platform.These three main axes form a "platform + pipeline" dual-drive model, reusing cross-project technical experience, improving R&D efficiency, and achieving precise delivery.
Apart from KY-0301, its cell therapy pipeline is also advancing, such as the clinical trial application for BMCA/CD19 dual-target CAR-T (KQ-2003) being accepted; in terms of macromolecules, KY-0118 (a biased IL-2 bifunctional fusion protein targeting PD-1) has entered the clinical stage and is exploring combination pathways with PD-L1 monoclonal antibodies.
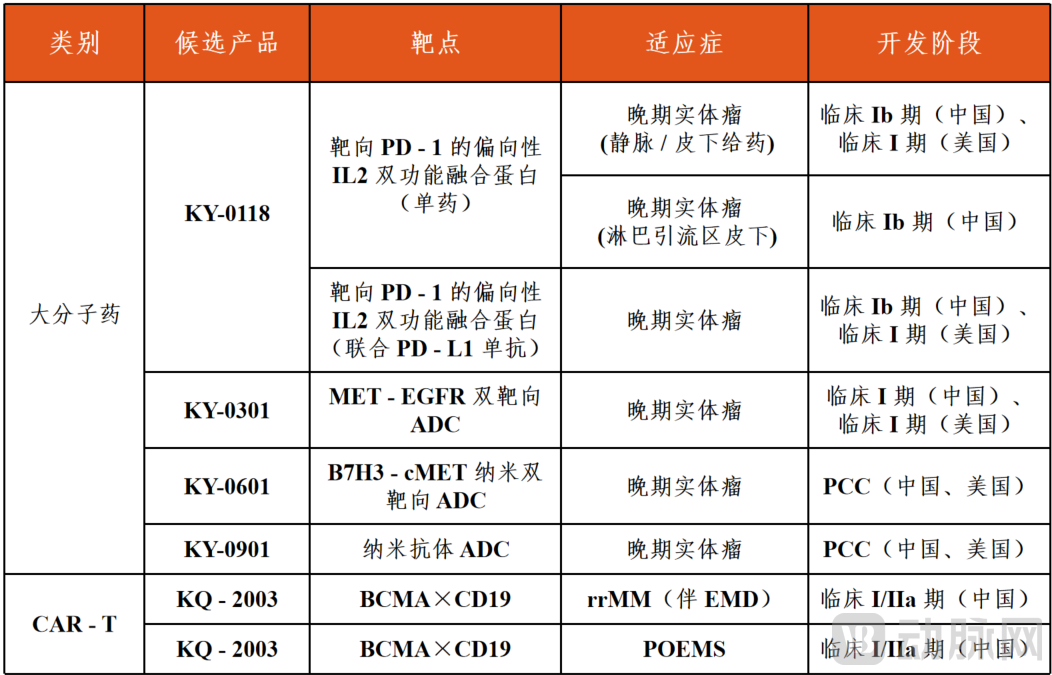
Overview of Keyi (Zhejiang) Pharmaceutical Technology Co., Ltd. Pipeline
ADC's overseas expansion continues to accelerate
In recent years, the ADC market has entered a period of rapid expansion.According to Frost & Sullivan, the global ADC market size is expected to rapidly increase from $7.9 billion in 2022 to $64.7 billion in 2030, with a compound annual growth rate (CAGR) of approximately 30% from 2022 to 2030; in China, the domestic ADC market is also maintaining high-speed growth, expected to expand from 800 million RMB in 2022 to 66.2 billion RMB in 2030, with a CAGR as high as 73%.
Behind the rapid expansion of the global and Chinese markets, the interest of capital and multinational pharmaceutical companies in ADCs continues to rise. In recent years, Chinese companies are gradually becoming key participants in this wave, with frequent overseas collaborations. This licensing deal, worth up to $1.165 billion, is not only a milestone for Keyi (Zhejiang) Pharmaceutical Technology Co., Ltd., but also a microcosm of the trend of China's ADC going global.
In the past few years, we have seen the amount involved in licensing deals gradually increase, with collaborations worth over 500 million US dollars no longer being rare. At the same time, the logic behind these deals has shifted from "having targets and data" to "having platforms, processes, and scalability." Like KY-0301, which not only demonstrates quantifiable highlights such as DAR uniformity and tissue penetration but also advances clinical trials simultaneously in China and the US, opening up broader prospects for going global.
Taking this licensing agreement as a pivotal reference, three key trends can be anticipated for China’s ADC global expansion phase 2.0: first, business development transactions will become more frequent and routine, with greater emphasis on verifiable engineering and clinical operability—such as drug-to-antibody ratio (DAR) homogeneity, quantitative evidence of tissue penetration/exposure, and safety margins; second, global clinical deployment will be initiated earlier, with concurrent dual reporting and approval strategies alongside cross-regional multicenter trials becoming critical to enhance valuation and shorten the time-value curve; third, capital strategies will grow more flexible, using licensing proceeds combined with domestic/overseas financing and listings as leverage, enabling companies to strengthen cycle-crossing capabilities through the integrated management of cash flow, valuation, and clinical data.
Looking back at this news: Radiance Biopharma obtains the exclusive overseas license for KY-0301, with a total consideration of up to $1.165 billion; Keyi (Zhejiang) Pharmaceutical Technology Co., Ltd., with its nano bispecific ADC platform (TPEBEN) and engineering highlights such as uniform DAR, along with dual IND submissions and approvals in both China and the US, as well as the progress of Phase I/II patient enrollment, provides a practical implementation foundation for international collaboration.The so-called "delivering medicine to the world" ultimately needs to be implemented in clinical and manufacturing processes, with verifiable data supporting every step of decision-making.
