[First Release]Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd. Completes Hundreds of Millions of Yuan in Series B Financing, Accelerating the Construction of a Full-Process Service Platform for Medical Devices
VCBeat learned at the first time that Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd. (hereinafter referred to as "Shuimu Zhonghui Medical"), a leading domestic full-process service platform for medical devices, officially announced the completion of a multi-billion-yuan strategic B-round financing. This round of financing was led byHony Capital、Guojv Venture CapitalCo-led byGuosheng Capital, Guangzhou Industrial Investment CapitalParticipate in investment,Xizhiqiao CapitalAct as the exclusive financial advisor. The funds raised this time will be used to expand the integrated service platform for medical devices and strengthen the construction of a nationwide service network.
Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd. is the first privately established third-party professional medical device inspection and testing institution in China. The core members of the team all have more than ten years of relevant professional experience, and its current business coverage includes"Engineering R&D + Testing & Inspection + Clinical Trials + Registration + CDMO (including IVD reference materials and quality control products R&D and production) + Market Access"A one-stop service platform for the entire medical device industry chain, capable of providing full lifecycle technical services from medical device productization to commercialization.Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd. adheres to the mission of "making it easy for everyone to develop medical devices" and truly achieves"Return R&D to R&D, and leave compliance to Shuimu."

Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd.'s testing laboratory holds dual national-level certifications of CMA and CNAS, and has obtained accreditation from the American Association for Laboratory Accreditation (A2LA) in the electrical field. Currently, the laboratory's testing capabilities cover more than 95% of active medical devices, in vitro diagnostics, as well as some passive testing projects. The core team has an average of over 15 years of industry experience, with long-term expertise in medical device regulations, standards, and testing methodologies. They are committed to supporting rapid market entry for enterprise products through "comprehensive testing scope and professional service systems."

Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd. and the University of Electronic Science and Technology of China jointly established the "Shuimu Medical-University of Electronic Science and Technology of China" Joint Laboratory, which boasts a strong research team and advanced R&D technology. The lab assists companies in the hardware and software co-development of products, reliability analysis, testing validation, and application evaluation, helping enterprises improve R&D efficiency while reducing costs.

The company's clinical CRO business has a strong industrial service platform internally and abundant industry resources externally. Its operations cover many fields such as radiotherapy equipment/systems, medical robots, IVD, medical aesthetics, cardiovascular/neurological interventions, and digital therapeutics, with a layout radiating across the country.

The platform is an innovative high-tech enterprise with core competitive resources in the research and development, production, and services of in vitro diagnostic-related products. From the R&D design of in vitro diagnostic products to the establishment of a quality management system, from providing full-process services for contract development and manufacturing organization (CDMO) to interpreting medical device laws and regulations, from creating China's own third-party internal quality control materials to achieving laboratory quality management control, Shuimu Jiheng aims to provide customers with comprehensive lifecycle solutions for in vitro diagnostics.
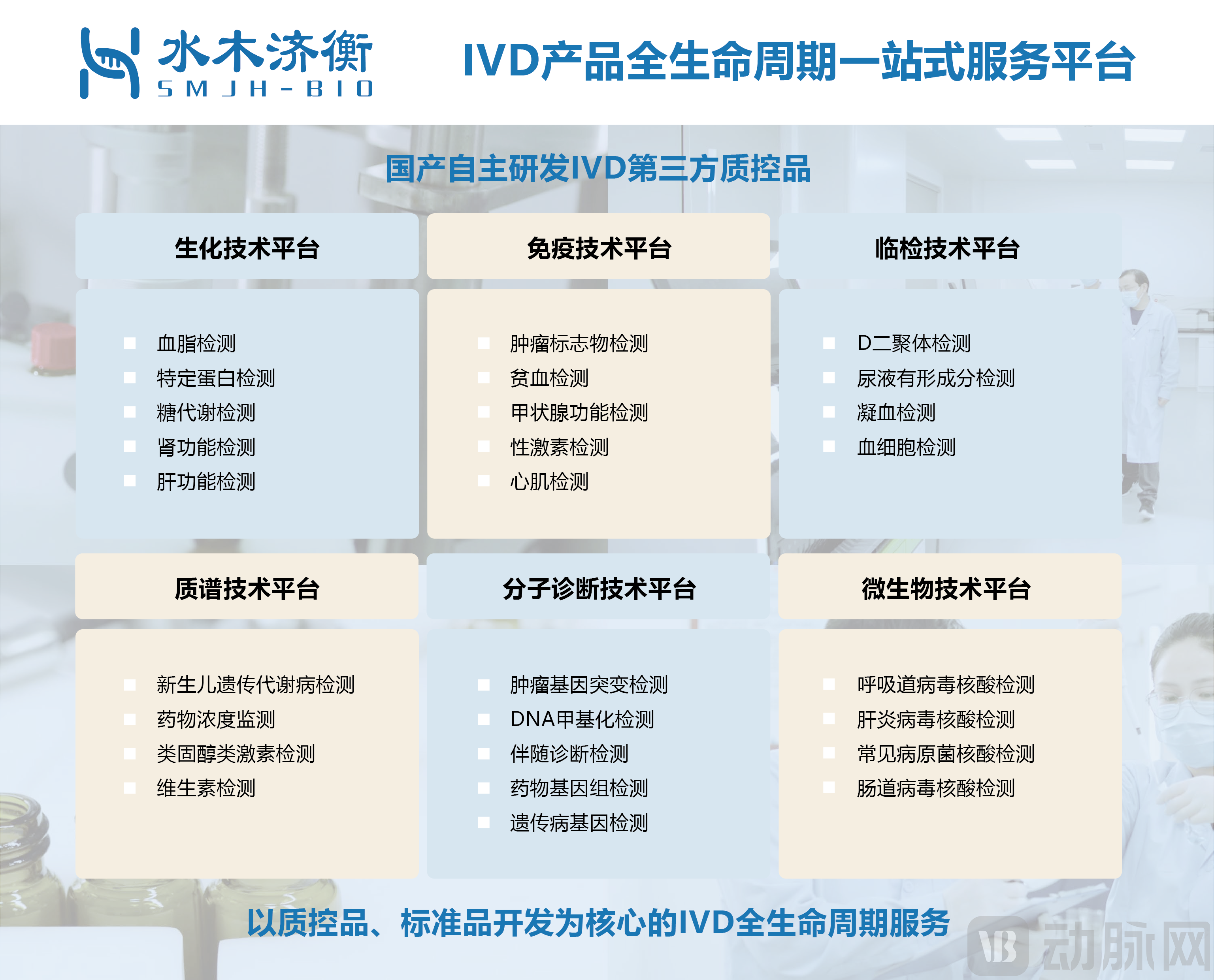
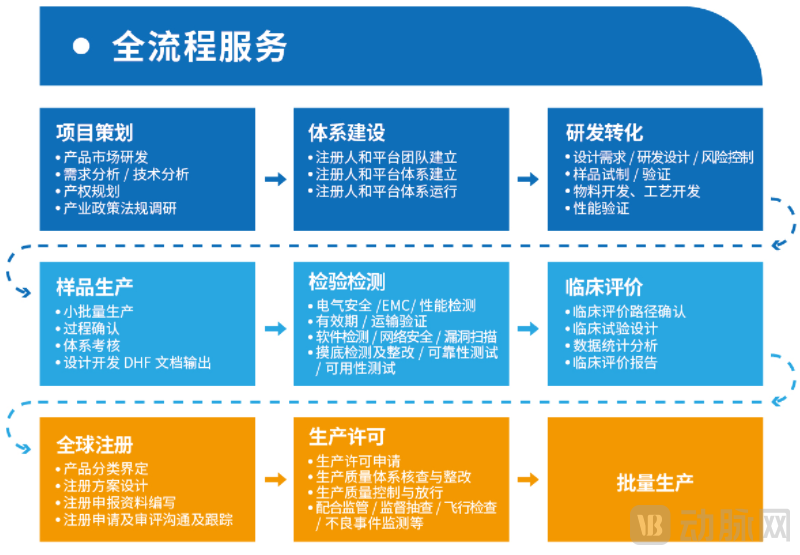
The company relies on industry-leading project experience, a strong execution team, and abundant resource accumulation. Operating in a dual "incubator + CDMO" model, it has built a one-stop CDMO service platform covering the entire range of medical device categories. Equipped with Class 10,000 and Class 100,000 cleanrooms, a sterile validation testing center, and a contract R&D and production platform, the company possesses full-category R&D and production capabilities, providing comprehensive end-to-end services for medical devices across the entire industry chain from concept validation, R&D design, pilot verification, to registration systems.
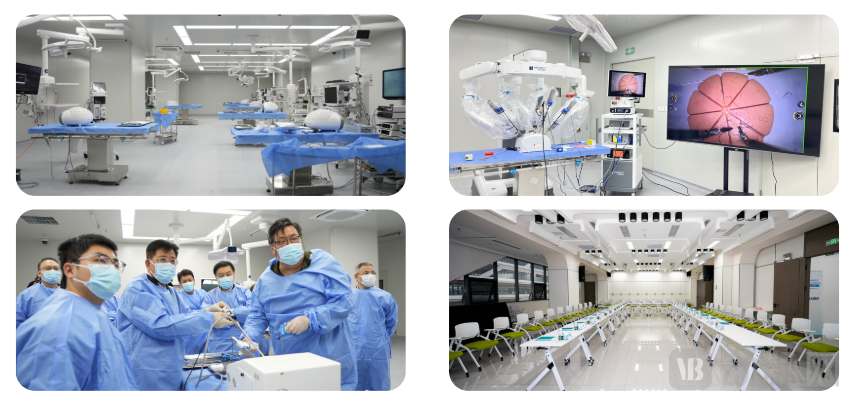
As China's first third-party simulated medical training center, the company has filled a critical gap in the domestic market with its advanced facilities and professional services. Its core business segments include: clinical application evaluation, medical insurance pricing and market access, medical training, and animal testing. The center features 12 fully simulated operating rooms replicating real clinical environments, along with functional areas such as imaging and laboratory departments. Equipped with over 500 real training devices and digital medical education solutions, it creates a highly immersive and clinically authentic teaching environment. The company has already established partnerships with multiple leading multinational corporations (MNCs) and possesses extensive resources spanning “clinical practice, education, research, and production.” By building an end-to-end service system covering “proof-of-concept, technology maturation, product iteration, and application promotion,” it deeply integrates clinical, engineering, and market resources to support companies in crossing the critical stages from R&D to commercialization.
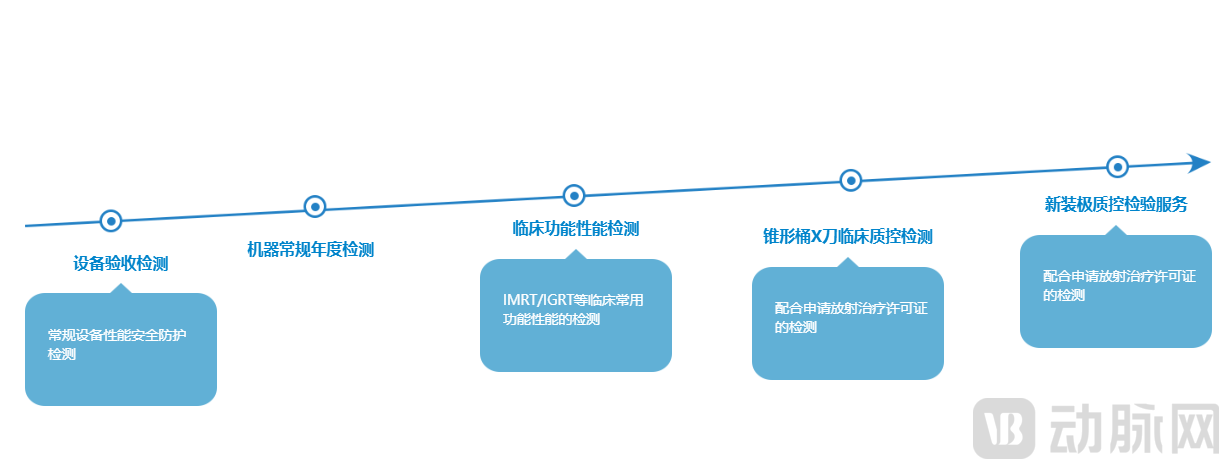
As a digital operation platform in the life science industry, the company's businesses cover testing services related to quality control in radiotherapy physics (Gamma Knife, linear accelerators, proton, heavy ion), and it can issue inspection reports certified by the state with the CMA mark. The company also provides one-stop services for building oncology radiotherapy disciplines, including bunker construction, third-party quality control products for radiotherapy, expert technical support, and remote target area contouring for radiotherapy.
Sun Jingsheng, Founder of Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd.The company’s unique full-industry-chain model addresses key bottlenecks in the industry from R&D to market entry. We also believe that with the continuous growth of China's medical device market and the ongoing innovation drive in domestic products, Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd.'s service model will enhance overall industry efficiency and play a role in promoting the listing of innovative medical devices, truly making "no difficult-to-make medical devices in the world." At this point, Shuimu will also complete its nationwide coverage across the Beijing-Tianjin-Hebei region, the Yangtze River Delta, the Pearl River Delta, and the southwestern region. It is hoped that Shuimu can promote the improvement of regional industrial chains and the development of industrial clusters.
Lang Xiaofeng, Managing Director of Hongyi InvestmentWe are optimistic about the business model of Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd.'s integrated service across the entire medical industry chain, and we value the company's experienced business team. This is another strategic move by Hony Capital in the ecosystem of the medical device industry chain. We look forward to leveraging the synergy of capital, resources, and industrial networks to help Shuimu Medical expand market boundaries while empowering the industry chain to support the development of more innovative medical device companies, creating value.
Yang Xi, Chairman of Guangzhou Guoju Venture Capital Co., Ltd.Guoju Venture Capital, as the core investment platform of Guangzhou High-tech Investment Group for the layout of the entire biopharmaceutical industry chain, has always focused on the goal of creating a "trillion-level biopharmaceutical industrial cluster" in the Guangzhou Development Zone. It actively introduces and nurtures high-quality enterprises that can integrate upstream and downstream parts of the industry chain. The one-stop service model for the entire life cycle of medical devices developed by Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd. will strongly promote the innovative, clustered, and ecological development of the medical device industry in Guangzhou and even the Greater Bay Area. We look forward to collaborating with Shuimu Medical to jointly build an innovation hub for medical devices in the Guangdong-Hong Kong-Macao Greater Bay Area.
Guosheng CapitalBeijing Shuimu Zhonghui Science and Technology Development Co., Ltd. has a professional talent team and a mature quality management system, building a high competitive barrier. This time, as the leading enterprise in the medical device service chain, its settlement in Chengdu High-tech Zone will fill the gap of Chengdu's full-process professional service platform for medical devices, attract more high-quality enterprises to settle down, build a rich network of medical device enterprises for Chengdu, and empower the development of Chengdu's medical device industry.
Guangzhou Industrial Investment CapitalBiomedical and healthcare industry is one of the five key industries in Guangzhou. Beijing Shuimu Zhonghui Science and Technology Development Co., Ltd., a leading domestic biomedical and healthcare platform enterprise, focuses on active medical devices, large-scale radiotherapy equipment, medical robots, AI medical devices, biomaterials, IVD, medical aesthetics, digital therapeutics and other fields. The establishment of Shuimu Medical in Guangzhou will help promote the city's inspection, testing, and professional service infrastructure, further strengthen critical aspects of medical device R&D, support Guangzhou in enhancing its high-end medical device industrial chain while radiating influence across the Guangdong-Hong Kong-Macao Greater Bay Area, and build a domestically influential high-end medical device industry cluster.
