J-VALVE TF: China's NMPA approves the first transfemoral retrograde TAVI device
Genesis MedTech announces that J-VALVE® TF Transcatheter Aortic Valve System (J-VALVE TF Valve), independently developed by its subsidiary Suzhou Jiecheng, has received China's National Medical Products Administration (NMPA) approval for market launch. The J-VALVE TF features a globally patented “movable positioning component design” and is currently the only interventional heart valve product approved by the NMPA for treating aortic valve regurgitation via a transvascular approach.
In China, the number of patients with severe aortic valve regurgitation is approximately twice that of patients with severe aortic stenosis (AS). Patients with aortic regurgitation (AR) generally have a poor quality of life and a high mortality rate with conservative treatment. Moreover, many AR patients cannot tolerate traditional surgical aortic valve replacement (SAVR). Transcatheter aortic valve implantation (TAVI) offers a new option for these patients who are surgically ineligible or high-risk. However, AR patients often have anatomical characteristics such as an oversized valve structure and leaflets lacking calcification, which result in a lack of anchoring zones for conventional prosthetic valves and pose significant challenges for implantation.
To address this issue, Genesis (Jiecheng) has developed J-VALVE TF, the first TAVI product for treating aortic regurgitation via the femoral artery based on clinical needs. Its innovative movable positioning component design can effectively address the shortcomings of previous products, which were difficult to anchor and prone to complications, significantly improving treatment outcomes for AR patients undergoing TAVI. This innovative design makes J-VALVE TF a true ‘first-in-class' in China's TAVI field. At the same time, it is the only Chinese TAVI that has gained recognition from industry giants like Edwards Lifesciences and U.S. Federal Trade Commission (FTC). Edwards Lifesciences may be interested in J-VALVE TF but has to abandon Trilogy, JenaValve Technology’s TAVI product for aortic regurgitation. As expected, the innovatively designed J-VALVE TF received NMPA approval for market launch through the Special Review Program for Innovative Medical Devices.
J-VALVE TF is an interventional valve product suitable for patients with severe aortic regurgitation (incompetence) and stenosis, offering a dual indication. The main difference between this product and the already marketed J-VALVE TA transapical valve is that J-VALVE TF is delivered via the transfemoral route, providing advantages such as shorter procedure time, less surgical trauma, and faster patient recovery.
J-VALVE TF: Composed of a bovine pericardial short-frame valve, a globally patented movable positioning component, and a 270° adjustable bending delivery system.
Globally patented movable positioning component locks the interventional valve in place using a dual vertical force, axial positioning force and radial support from the valve frame. It provides bidirectional anchoring without requiring excessive oversizing, significantly reducing the incidence of atrioventricular conduction block. The movable linkage positioning system better captures the leaflets, enabling automatic sinus entry and self-alignment, better protecting the coronary arteries, accommodating more patients, and reducing complication rates.
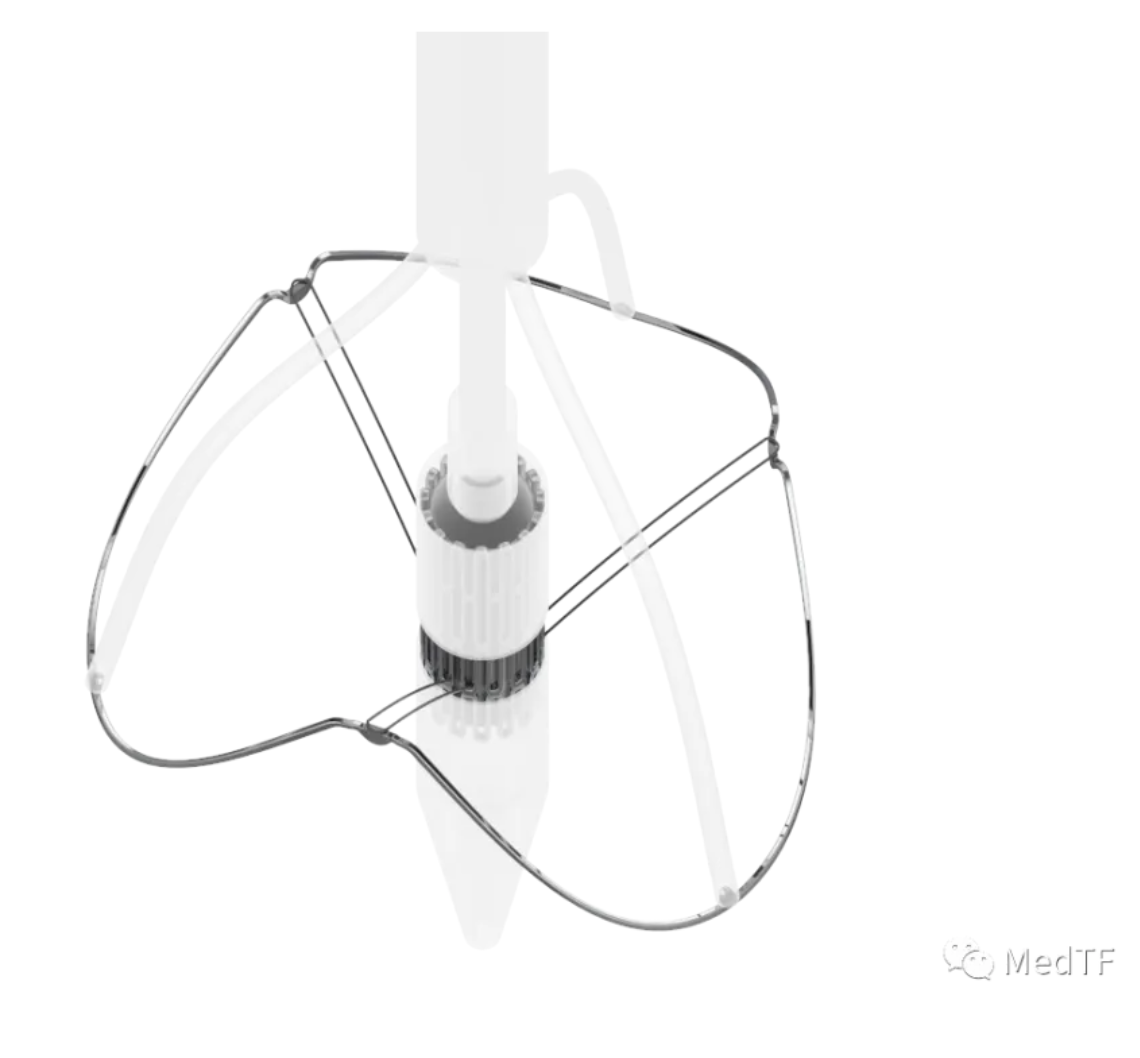
Bovine pericardial short-frame valve uses bovine pericardial leaflets and exclusive anti-calcification biovalve technology, demonstrating good durability (transapical J-VALVE ten-year reintervention rate: 3.7%). Coronary protection device can prevent coronary obstruction through the “crown-shaped” short-frame design and by using the positioning component to press the leaflets down. Valve sizes range from 21 to 34 mm


Genesis MedTech is a medical device company dedicated to building an open medical technology platform and establishing an innovation network worldwide. Based on an open innovation platform, Genesis actively integrates Chinese and international resources, promotes upstream and downstream collaboration, and continuously develops a diverse range of cost-effective products tailored to the needs of Chinese patients.
Source: MedTF
