The sixth domestically produced hyaluronic acid "hydrodermic needle" has been officially approved.
As a form of "mesotherapy," hydrodermabrasion effectively addresses the issue of skincare products struggling with transdermal absorption by precisely delivering nutrients into the superficial or middle layers of the dermis. The primary ingredient, hyaluronic acid, is an extracellular matrix component widely distributed in human and animal tissues such as the dermis, lens, and articular cartilage. Due to its material properties, it can also serve purposes like filling facial depressions and providing skin hydration and moisture

September 1, 2025, Dalian Fullerene Pharmaceutical Co., Ltd""Sodium Hyaluronate Injection Solution"Officially approved for market release by the National Medical Products Administration, with registration certificate number: State Medical Device Approval 20253131774,The product is primarily used for injection into the superficial layer of the facial dermis.Temporarily improves dry skin and dull complexion in adults.。At the same time, the packaging structure is a vial, with the solution composedNon-crosslinked hyaluronic acid, sodium chloride, sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dodecahydrate, and water forComposed of.

Domestic Hyaluronic Acid Hydrating Facial
Six products approved for Class III certificates
PART 01
MesIt generally refers to injectable hydro-boosting treatments, administered in the superficial to middle layers of the dermis, which provide significant hydrating and moisturizing effects. Hydro-boosting injections primarily consist of a sodium hyaluronate-based solution, with the main purpose of...Hydrating and moisturizing, while also shrinking pores and improving skin tone.Equivalent
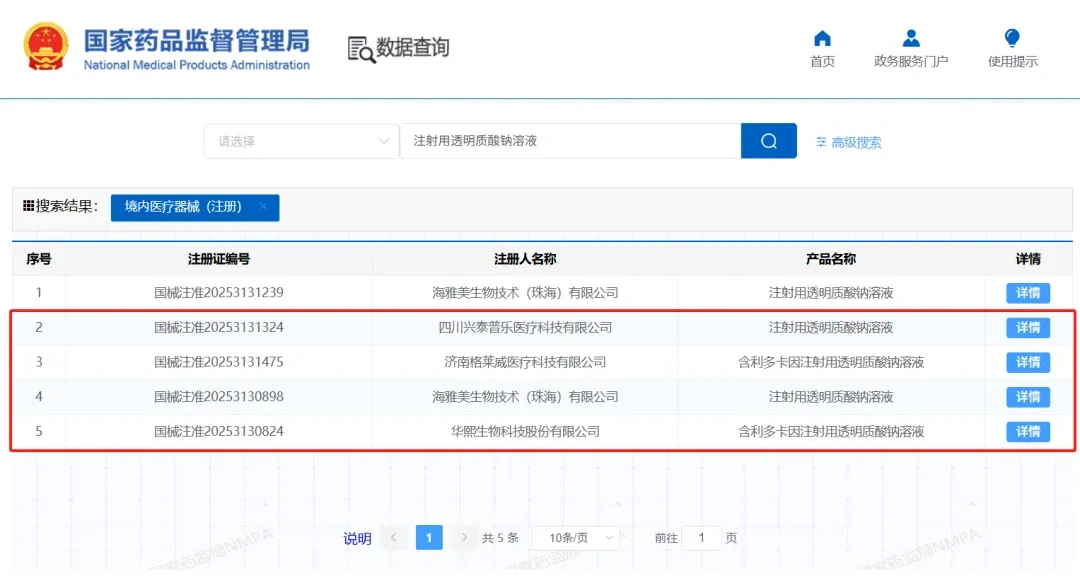
As of now, there are five approved dermal fillers in the domestic market that comply with regulations, including those from Bloomage BiotechRunzhi® Titou,润百颜® Bolo (with anesthetic), Swiss Hyamax's Clara Hepburn, Lepu Medical's Flora®, Glavive's Sodium Hyaluronate Injection Solution (with anesthetic), and Dalian Fullerene's newly approved aqua-shine products。
Dalian Fullerene
Create a medical beauty and
PART 02
Dalian Fullerene Pharmaceutical Co., Ltd. was established in 2019 and is affiliated with Dongxing (D) A subsidiary of the Health Industry Group. The company is primarily engaged inPharmaceutical production and wholesale, medical device operation and production, cosmetics research and development andand other businesses, holds over 50 Class II medical device approvals, covers full-category production qualifications for medical devices/cosmetics/disinfectants/food designations, possesses a 10,000-grade GMP workshop and a 30-person R&D team, and has applied for more than 30 national invention


As of now, the fullerene product matrix has coveredWhitening, anti-aging,Three core areas with a rich and diverse product portfolio, coveringMesoderm, Medical Repair, Homeand other series, with star products like Runbai and Xuanbai highly favored by the market, providing comprehensive and personalized whitening solutions for beauty seekers with diverse

